TL;DR
Paroxysmal cold hemoglobinuria is a rare autoimmune hemolytic anemia caused by the Donath-Landsteiner (DL) antibody.
- DL antibody (IgG) binds to red blood cells (RBCs) in cold temperatures. Upon warming, complement activation leads to intravascular hemolysis via the membrane attack complex (MAC).
- Often triggered by viral infections, especially in children.
- Classic triad: Hemoglobinuria (dark urine), back pain, chills/fever after cold exposure.
- Can also include abdominal pain, jaundice, fatigue, and renal involvement.
- Positive Donath-Landsteiner test (gold standard).
- Direct antiglobulin test (DAT) positive for complement (C3).
- Hemoglobinuria and hemosiderinuria.
- Elevated LDH, decreased haptoglobin.
- Avoidance of cold exposure (primary).
- Treatment of underlying infections.
- Supportive care (transfusions, hydration).
- Immunosuppressive therapy (corticosteroids, rituximab).
- Complement inhibitors (eculizumab, ravulizumab) for severe cases.
Prognosis and Complications ▾:
- Prognosis varies; generally good for acute PCH, more variable for chronic PCH.
- Potential complications include renal failure, chronic anemia, and rare thrombotic events.
*Click ▾ for more information
Introduction
Paroxysmal cold hemoglobinuria (PCH) represents a rare yet significant autoimmune hemolytic anemia (AIHA) characterized by the immune system’s aberrant production of antibodies that target and destroy red blood cells. This destruction is uniquely triggered by exposure to cold temperatures, setting paroxysmal cold hemoglobinuria (PCH) apart from other forms of hemolytic anemia.
The hallmark of paroxysmal cold hemoglobinuria (PCH) involves the body’s response to cold, leading to the destruction of red blood cells and the subsequent release of hemoglobin into the bloodstream and urine.
Pathophysiology of Paroxysmal Cold Hemoglobinuria
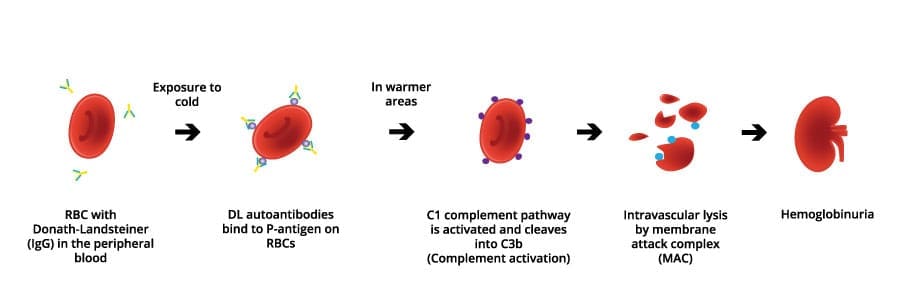
The core of paroxysmal cold hemoglobinuria (PCH) lies in the unique behavior of the Donath-Landsteiner (DL) antibody. This antibody exhibits a biphasic behavior: it binds to the surface of red blood cells at low temperatures, primarily targeting the P-antigen on red blood cell (RBC) surfaces.
Upon subsequent warming of the blood as it circulates back to the body’s core, this antibody triggers the activation of the complement system, a crucial part of the immune response. This complement activation culminates in intravascular hemolysis, the rupture and destruction of red blood cells within the blood vessels.
As the red blood cells are destroyed, hemoglobin is released into the bloodstream. This free hemoglobin is then filtered by the kidneys and excreted in the urine, leading to a characteristic darkening or red discoloration of the urine, a phenomenon known as hemoglobinuria.
Paroxysmal cold hemoglobinuria is also referred to as Donath-Landsteiner hemolytic anemia (DLHA) or Donath-Landsteiner syndrome, names derived from the researchers who first described this condition and its unique antibody . The term “paroxysmal” signifies the sudden, episodic nature of the hemolytic attacks, often precipitated by cold exposure, while “cold” emphasizes the temperature-dependent trigger, distinguishing it from other hemolytic anemias.
Comparison to other AIHAs and PNH
| Feature | Paroxysmal Cold Hemoglobinuria (PCH) | Warm Autoimmune Hemolytic Anemia (Warm AIHA) | Cold Agglutinin Disease (CAD) | Paroxysmal Nocturnal Hemoglobinuria (PNH) |
| Cause | Autoimmune (Donath-Landsteiner antibody) | Autoimmune | Autoimmune | Acquired genetic mutation in hematopoietic stem cells |
| Antibody/Mechanism | IgG (Donath-Landsteiner), biphasic, complement-mediated intravascular hemolysis | IgG, extravascular hemolysis (spleen) | IgM, RBC agglutination, complement activation | Deficiency of GPI-anchored proteins (CD55, CD59), uncontrolled complement activation |
| Target Antigen | P antigen on RBCs | Variable RBC antigens (often Rh system) | I/i antigens on RBCs | N/A (deficiency of protective proteins) |
| Temperature Dependence | Yes (cold-induced binding, warm-induced hemolysis) | No (reacts at body temperature) | Yes (agglutination at cold temperatures) | No (constant complement susceptibility) |
| Hemolysis | Intravascular | Extravascular | Variable (mostly extravascular) | Intravascular |
| Clinical Presentation | Hemoglobinuria (after cold exposure), back pain, chills, fever | Fatigue, pallor, jaundice, splenomegaly | Acrocyanosis, Raynaud’s phenomenon, mild anemia | Hemoglobinuria (especially nocturnal), thrombosis, pancytopenia |
| Laboratory Findings | Positive Donath-Landsteiner test, DAT (C3), hemoglobinuria, hemosiderinuria | DAT (IgG, +/- C3), anemia, reticulocytosis | DAT (C3), high cold agglutinin titer | Flow cytometry (CD55/CD59 deficiency) |
| Typical Patient Population | Children (post-viral), adults | Adults | Older adults | Adults |
| Key Diagnostic Test | Donath-Landsteiner test | Direct antiglobulin test (DAT) | Cold agglutinin titer, DAT. | Flow cytometry. |
| Treatment | Avoid cold, supportive care, immunosuppression, complement inhibitors. | Corticosteroids, rituximab, splenectomy. | Avoid cold, rituximab. | Eculizumab, ravulizumab, bone marrow transplant. |
Causes of Paroxysmal Cold Hemoglobinuria (PCH)
While the core mechanism of paroxysmal cold hemoglobinuria involves the Donath-Landsteiner (DL) antibody, the underlying triggers that lead to its production can vary.
- Post-Infectious PCH: This is the most common cause, particularly in children.
- Viral Infections: Measles, mumps, varicella (chickenpox), Epstein-Barr virus (EBV), cytomegalovirus (CMV), and other viral infections have been implicated. The exact mechanism is not fully understood, but it’s thought that molecular mimicry plays a role. This means that viral antigens may share structural similarities with the P antigen on red blood cells (RBCs), leading to the production of cross-reactive antibodies.
- Bacterial Infections: Historically, syphilis was a significant cause of paroxysmal cold hemoglobinuria (PCH). While much less common now, other bacterial infections can also trigger the condition.
- Idiopathic PCH: In a substantial number of cases, no identifiable preceding infection or underlying cause can be found.
- Autoimmune Disorders: In rare instances, paroxysmal cold hemoglobinuria (PCH) can be associated with other autoimmune disorders. The relationship is not fully understood, but immune dysregulation is a common factor.
- Underlying conditions: Although rare, there are instances where paroxysmal cold hemoglobinuria (PCH) appears after other conditions, but the relationship is not always clear.
Signs and Symptoms of Paroxysmal Cold Hemoglobinuria
- Hemoglobinuria: This is the hallmark symptom. It manifests as dark or reddish-brown urine, particularly after exposure to cold. The color change is due to the presence of free hemoglobin in the urine, released from hemolyzed red blood cells (RBCs). The degree of hemoglobinuria can vary, depending on the severity of hemolysis.
- Back Pain: Often described as a severe, aching pain in the lower back. It is thought to be related to the acute hemolysis and the release of inflammatory mediators.
- Chills and Fever: These symptoms are also associated with the acute hemolytic episodes. They can range from mild shivering to high-grade fevers.
- Abdominal Pain: Some patients may experience abdominal cramping or discomfort. This can be due to the systemic effects of hemolysis. Gastrointestinal symptoms such as nausea, vomiting, and diarrhea have also been reported.
- Jaundice: In cases of significant hemolysis, jaundice (yellowing of the skin and eyes) may occur. This is due to the increased production of bilirubin, a byproduct of hemoglobin breakdown.
- Fatigue and Weakness: Chronic anemia resulting from recurrent hemolytic episodes can lead to fatigue, weakness, and pallor. Headache and a general feeling of discomfort, uneasiness, or illness (malaise) are also common complaints.
Less common symptoms
- Raynaud’s Phenomenon: In some individuals, particularly those with underlying autoimmune conditions, Raynaud’s phenomenon (episodes of reduced blood flow to the fingers and toes in response to cold) may be present.
- Renal Involvement: In severe cases, the kidneys can be affected by the large amounts of free hemoglobin. This can lead to acute kidney injury or renal failure. Signs of renal involvement may include decreased urine output, edema (swelling), and elevated creatinine levels
- Skin rash or urticaria (hives): Reddening and itching of the skin also triggered by cold exposure .
- Signs of shock, including altered mental status, rapid heartbeat (tachycardia), and metabolic acidosis, may also occur in individuals experiencing profound hemolysis.
Symptoms in Children
Paroxysmal cold hemoglobinuria (PCH) is more common in children, often following viral infections. Children may present with similar symptoms as adults, but the onset can be more abrupt and severe. They also may have more significant fever.
Acute vs Chronic Paroxysmal Cold Hemoglobinuria
| Feature | Acute PCH | Chronic PCH |
| Typical Trigger | Acute infection (viral most common) | Repeated cold exposure |
| Onset | Abrupt | Recurrent episodes |
| Duration | Transient (days to weeks), resolves with infection | Recurrent, long-term |
| Underlying Cause | Post-infectious | Historically chronic infection (syphilis), now primarily idiopathic (rare) |
| Donath-Landsteiner Antibody | Transient presence | May be persistent |
| Symptoms | Severe; hemoglobinuria, back pain, chills, fever | Recurrent hemoglobinuria and related symptoms upon cold exposure |
| Anemia | Rapid and significant onset | May lead to chronic anemia |
| Typical Patient Population | Children, often post-viral | Historically adults, now very rare |
| Frequency | Single event, tied to infectious episode | Recurrent episodes upon cold exposure |
| Prognosis | Generally resolves with treatment of underlying infection | Variable, dependent on severity and cold avoidance |
Laboratory Investigations
The diagnosis of paroxysmal cold hemoglobinuria relies heavily on laboratory investigations that confirm the presence of hemolysis and specifically identify the characteristic Donath-Landsteiner antibody.
Absolutely. Accurate laboratory investigations are crucial for confirming a diagnosis of Paroxysmal Cold Hemoglobinuria (PCH). Here’s a detailed breakdown of the necessary tests and their expected findings:
- Complete Blood Count (CBC): A complete blood count (CBC) typically reveals anemia, indicated by a low red blood cell count . In the acute phase, the bone marrow’s response may be delayed, leading to a low number of immature red blood cells (reticulocytopenia). In the chronic phase, reticulocytosis indicates the bone marrow’s attempt to compensate for RBC destruction. Possible leukocytosis or leukopenia, depending on any underlying infection.
- Peripheral Blood Smear: Peripheral blood smear might show spherocytes, which are abnormally shaped red blood cells indicative of hemolysis, and polychromasia, if the bone marrow has begun to increase red blood cell production.
- Urinalysis: Urinalysis is crucial and often reveals hemoglobinuria. Positive for blood on dipstick, but microscopic examination may reveal few or no red blood cells. The urine will appear dark red, or brown. In chronic cases, hemosiderinuria, the presence of iron in the urine, may also be detected.
- Direct Antiglobulin Test (DAT/Coombs Test): Typically positive for polyspecific DAT, primarily due to complement (C3) binding to RBCs. Positive for C3 in monospecific DAT, but often negative or weakly positive for IgG. This reflects the complement-mediated hemolysis characteristic of paroxysmal cold hemoglobinuria (PCH).
The indirect antiglobulin test may be positive, and further testing can determine the specificity of the antibody, which in paroxysmal cold hemoglobinuria (PCH) is typically anti-P .
- Donath-Landsteiner Test: This is the gold standard for diagnosing paroxysmal cold hemoglobinuria (PCH). This specialized in vitro assay is designed to detect the presence of the characteristic biphasic Donath-Landsteiner antibody.
The procedure involves incubating the patient’s serum with red blood cells that express the P-antigen under specific temperature conditions. Typically, three sets of tubes are prepared. The first set contains patient serum and P-antigen positive red blood cells, which is incubated at a cold temperature (0-4°C) for a period, followed by warming to 37°C. The second set is incubated only at the cold temperature, and the third set only at 37°C, serving as controls.
A positive DL test is indicated by hemolysis (rupturing of red blood cells) observed only in the tube that was initially incubated in the cold and subsequently warmed to body temperature.
Proper handling of the blood sample is critical for accurate results; the sample should be collected and maintained at 37°C during transport to prevent the antibody from binding to red blood cells prematurely, which could lead to a false negative result. False negative results can also occur due to the transient nature of the antibody or incorrect specimen handling.
- Biochemical Markers of Hemolysis:
- Elevated lactate dehydrogenase (LDH): Released from damaged RBCs.
- Decreased haptoglobin: Haptoglobin binds free hemoglobin, so levels decrease during hemolysis.
- Elevated indirect bilirubin: A byproduct of hemoglobin breakdown.
- Renal Function Tests: Elevated creatinine and blood urea nitrogen (BUN) in cases of renal involvement. These tests will help assess any kidney damage.
- Cold Agglutinin Titer: This is used to differentiate paroxysmal cold hemoglobinuria (PCH) from Cold Agglutinin Disease (CAD). In paroxysmal cold hemoglobinuria (PCH), the cold agglutinin titer is usually low or negative, while in CAD, it is significantly elevated.
- Flow Cytometry: This is not a routine test for paroxysmal cold hemoglobinuria (PCH), but is used to rule out paroxysmal nocturnal hemoglobinuria (PNH). This test is used to detect the absence of CD55 and CD59. In PCH, these CD55, CD59 markers will be normal. This is important for differential diagnosis.
If an underlying cause for paroxysmal cold hemoglobinuria (PCH) is suspected, further investigations may be warranted. Serological tests for syphilis, such as the Treponema pallidum antibody test, should be performed, especially in adults with chronic paroxysmal cold hemoglobinuria (PCH).
Serological tests or polymerase chain reaction (PCR) assays for various viral infections (e.g., Epstein-Barr virus, cytomegalovirus, measles, mumps) may be conducted, particularly in children with acute paroxysmal cold hemoglobinuria (PCH).
Blood cultures may be indicated to identify bacterial infections like Mycoplasma pneumoniae or Haemophilus influenzae.
In adult patients with unexplained paroxysmal cold hemoglobinuria (PCH), especially those with chronic or relapsing symptoms, evaluation for underlying hematological malignancies, such as through a bone marrow biopsy or lymph node biopsy if lymphadenopathy is present, should be considered. Identifying and treating any underlying condition is crucial for the management of secondary paroxysmal cold hemoglobinuria (PCH).
Summary of the laboratory investigations of PCH
| Test | Typical Finding in PCH | Significance |
| CBC | Anemia (low RBC count), possible reticulocytopenia | Indicates red blood cell destruction and bone marrow response |
| Peripheral Blood Smear | Spherocytes, polychromasia (if reticulocytosis present) | Morphological evidence of hemolysis |
| Bilirubin (Indirect) | Elevated | Product of heme breakdown due to red blood cell lysis |
| LDH | Elevated | Enzyme released from damaged red blood cells |
| Haptoglobin | Low or Absent | Binds free hemoglobin; decreased levels indicate increased red blood cell destruction |
| Urinalysis | Hemoglobinuria (positive heme, few/no RBCs) | Direct evidence of hemoglobin in the urine due to intravascular hemolysis |
| Donath-Landsteiner Test | Positive (hemolysis at cold then warm) | Pathognomonic for PCH; detects the biphasic autoantibody |
| DAT (Direct Coombs) | Negative for IgG, usually positive for C3d | Suggests complement-mediated hemolysis without significant IgG binding (though variations exist) |
| Cold Agglutinin Titer | Usually not significantly elevated | Helps differentiate from cold agglutinin disease |
| Syphilis Serology | Positive if PCH is secondary to syphilis | Identifies syphilis as the underlying cause |
| Viral Serology/PCR | Positive if PCH is post-viral | Identifies a recent viral infection as a potential trigger |
| Bone Marrow Biopsy (Adults) | May show findings suggestive of underlying malignancy | Evaluates for hematological malignancies in adults with unexplained PCH |
Differential Diagnosis of PCH
| Condition | Key Symptoms | Key Laboratory Findings | Key Distinguishing Features |
| Paroxysmal Cold Hemoglobinuria (PCH) | Hemoglobinuria (after cold), back pain, chills, fever, abdominal pain, jaundice, fatigue | Positive Donath-Landsteiner test, DAT (C3+), hemoglobinuria, hemosiderinuria, elevated LDH, decreased haptoglobin | Cold-induced hemolysis, Donath-Landsteiner antibody, biphasic hemolysis |
| Cold Agglutinin Disease (CAD) | Acrocyanosis, Raynaud’s phenomenon, mild anemia, cold-induced symptoms | DAT (C3+), high cold agglutinin titer, RBC agglutination at cold temperatures | IgM-mediated agglutination, high cold agglutinin titer, less severe hemolysis |
| Warm Autoimmune Hemolytic Anemia (Warm AIHA) | Fatigue, pallor, jaundice, splenomegaly | DAT (IgG+), anemia, reticulocytosis | IgG-mediated hemolysis at body temperature, no cold sensitivity |
| Paroxysmal Nocturnal Hemoglobinuria (PNH) | Nocturnal hemoglobinuria, thrombosis, pancytopenia, abdominal pain | Flow cytometry (CD55/CD59 deficiency) | Clonal stem cell disorder, lack of GPI-anchored proteins, thrombosis risk |
| Rhabdomyolysis | Muscle pain, dark urine, muscle weakness | Elevated creatine kinase (CK), urine myoglobin, no hemoglobinuria on microscopic exam | Muscle breakdown, myoglobinuria, elevated CK |
| G6PD Deficiency | Hemolytic anemia after triggers (drugs, infections), jaundice, fatigue | Low G6PD enzyme activity | Enzyme deficiency, triggered by specific substances, not cold |
| Renal Causes of Hematuria (e.g., kidney stones, glomerulonephritis) | Hematuria (visible or microscopic), flank pain, dysuria, edema | Microscopic hematuria, abnormal renal function tests, renal imaging abnormalities | Renal origin of blood in urine, no systemic hemolysis |
Treatment and Management of Paroxysmal Cold Hemoglobinuria
The primary goals of treatment for paroxysmal cold hemoglobinuria are to provide supportive care and prevent further episodes of hemolysis by minimizing exposure to cold temperatures.
- Avoidance of Cold Exposure: This is the cornerstone of PCH management. Patients should avoid exposure to cold temperatures, including:
- Wearing warm clothing, especially gloves, scarves, and hats.
- Maintaining a warm indoor environment.
- Avoiding cold drinks and foods.
- Being cautious during cold weather or when entering air-conditioned spaces.
- Treatment of Underlying Infections: If PCH is triggered by an infection (e.g., viral or bacterial), prompt and appropriate treatment of the infection is essential. This may involve antiviral or antibiotic therapy. Treating the underlying infection can often lead to resolution of the PCH.
- Supportive Care:
- Transfusions: In cases of severe anemia, blood transfusions may be necessary to maintain adequate hemoglobin levels. Transfusions should be warmed to avoid triggering hemolysis. Due to the temperature-dependent nature of the Donath-Landsteiner antibody’s activity, P-antigen negative blood is typically not required; however, it may be considered in rare cases of refractory hemolysis despite other supportive measures. Pre-transfusion compatibility testing is usually not problematic as the antibody does not typically react at normal body temperatures.
- Folate Supplementation: Folate is essential for red blood cell production, and supplementation may be needed to support erythropoiesis during hemolytic episodes.
- Hydration:Maintaining adequate hydration is important to support renal function and minimize the risk of kidney damage from hemoglobinuria.
- Immunosuppressive Therapy:
- Corticosteroids: May be used to suppress the immune system and reduce antibody production. Often used in cases where cold avoidance alone is insufficient.
- Rituximab: A monoclonal antibody that targets B cells, which produce antibodies. May be used in refractory cases or when corticosteroids are ineffective.
- Other Immunosuppressants: In rare, severe cases, other immunosuppressants might be needed.
- Complement Inhibitors:
- Eculizumab and Ravulizumab: Monoclonal antibodies that inhibit the complement cascade. These drugs can be very effective in preventing hemolysis by blocking the complement pathway. These are used in cases with severe hemolysis, and in patients that don’t respond to other therapies.
- Monitoring and Follow-up: Regular monitoring of hemoglobin levels, renal function, and other relevant laboratory parameters is essential. Long-term follow-up is important to assess the effectiveness of treatment and monitor for potential complications. Patients should be educated about the signs and symptoms of hemolytic episodes and when to seek medical attention.
- Management of Complications:
- Renal failure: Requires careful management of fluid balance, electrolyte abnormalities, and potential dialysis.
- Thrombotic events: If they occur, require anticoagulation therapy.
Prognosis and Complications of Paroxysmal Cold Hemoglobinuria
Prognosis
The prognosis for paroxysmal cold hemoglobinuria varies depending on whether it presents as an acute or chronic condition.
- Acute PCH: The prognosis for acute PCH, especially in children following a viral infection, is generally good. With proper management of the underlying infection and supportive care, the hemolytic episodes usually resolve, and the Donath-Landsteiner antibody often disappears. Long-term complications are rare in these cases.
- Chronic PCH: The prognosis for chronic PCH is more variable and depends on the severity of hemolysis and the effectiveness of cold avoidance. With strict adherence to cold avoidance measures, many patients can lead relatively normal lives.
However, recurrent hemolytic episodes can lead to chronic anemia and potential complications. With the newer complement inhibitor medications, the prognosis has improved for those with severe chronic cases.
Overall, the prognosis for patients with PCH is generally considered excellent when the condition is promptly diagnosed and managed with appropriate supportive care. Mortality associated with PCH is rare and most often occurs due to multi-organ failure resulting from severe anemia secondary to massive acute hemolysis.
Potential Complications
- Renal Failure: Severe hemolytic episodes can lead to the accumulation of free hemoglobin in the kidneys, causing acute kidney injury or chronic renal failure. This is a serious complication and requires careful management of fluid balance and electrolyte abnormalities. In severe cases, dialysis may be necessary.
- Chronic Anemia: Recurrent hemolytic episodes can lead to chronic anemia, resulting in fatigue, weakness, and decreased quality of life. Long-term anemia can also contribute to cardiovascular complications.
- Thrombotic Events: Although less common than in PNH, there is a slightly increased risk of thrombotic events in PCH due to the release of procoagulant factors during hemolysis. This can lead to deep vein thrombosis, pulmonary embolism, or other thromboembolic complications.
- Iron Overload (Hemosiderosis): Frequent blood transfusions, which are sometimes necessary in severe PCH, can lead to iron overload. Iron overload can damage various organs, including the liver, heart, and endocrine glands.
Frequently Asked Questions (FAQs)
What is the difference between paroxysmal cold hemoglobinuria and PNH?
Although the names may sound similar, PCH and PNH are distinct conditions with different underlying causes and clinical presentations. PCH is an autoimmune hemolytic anemia where an antibody causes red blood cell destruction upon cold exposure, while PNH is a rare, acquired genetic disorder where red blood cells lack protective proteins, making them vulnerable to complement-mediated destruction. Therefore, PCH is caused by an autoantibody, and PNH is caused by a genetic mutation in blood stem cells. Also PCH is triggered by cold, whereas PNH is not.
What is the difference between PCH and cold agglutinin?
Paroxysmal cold hemoglobinuria (PCH) and cold agglutinin disease (CAD) are both cold-reactive hemolytic anemias, but they differ significantly: PCH is caused by a biphasic IgG antibody (Donath-Landsteiner) that triggers complement-mediated red blood cell lysis upon warming after cold exposure, leading to hemoglobinuria, while CAD is primarily caused by IgM antibodies that cause red blood cell agglutination (clumping) at colder temperatures, often resulting in acrocyanosis and milder, mostly extravascular hemolysis. Therefore PCH causes lysis of red blood cells, and CAD causes agglutination.
What is the most common cause of hemoglobinuria?
It is important to distinguish between hematuria (red blood cells in the urine) and hemoglobinuria (hemoglobin in the urine). While both can cause red or brown urine, they have different causes. That being said, when considering the most common causes that lead to hemoglobinuria, it is important to remember that it is caused by the breakdown of red blood cells. Therefore, the causes are related to that.
- Hemolytic anemias: This is a broad category of conditions where red blood cells are destroyed prematurely. This can include various types of autoimmune hemolytic anemias, as well as genetic conditions like sickle cell anemia and thalassemia.
- Transfusion reactions: Incompatible blood transfusions can cause rapid and severe hemolysis.
- Severe infections: Certain infections, such as malaria, can cause significant red blood cell destruction.
- Severe physical exertion: In some cases, intense physical activity can lead to red blood cell breakdown.
- Trauma: Severe trauma, especially crush injuries, can cause red blood cell destruction.
- Burns: Severe burn injuries can also cause red blood cell damage.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Shanbhag S, Spivak J. Paroxysmal cold hemoglobinuria. Hematol Oncol Clin North Am. 2015 Jun;29(3):473-8. doi: 10.1016/j.hoc.2015.01.004. Epub 2015 Mar 7. PMID: 26043386.
- Jacobs JW, Figueroa Villalba CA, Booth GS, Woo JS, Stephens LD, Adkins BD. Clinical and epidemiological features of paroxysmal cold hemoglobinuria: a systematic review. Blood Adv. 2023 Jun 13;7(11):2520-2527. doi: 10.1182/bloodadvances.2022009516. PMID: 36716137; PMCID: PMC10248093.
- Cooling LL. Kids, colds, and complement: paroxysmal cold hemoglobinuria. Transfusion. 2017 Jun;57(6):1332-1335. doi: 10.1111/trf.14128. PMID: 28594143.
- Radhakrishnan K, Downie PA, Earley CM. Paroxysmal Cold Hemoglobinuria: Diagnosis From the Blood Smear. J Pediatr Hematol Oncol. 2022 Mar 1;44(2):60-61. doi: 10.1097/MPH.0000000000002401. PMID: 35082245.
- Hogan KO, Oroszi G. Paroxysmal cold hemoglobinuria: A diagnostic dilemma in a paediatric patient. Transfus Med. 2023 Oct;33(5):416-419. doi: 10.1111/tme.12991. Epub 2023 Aug 13. PMID: 37574257.
- Lau-Braunhut SA, Stone H, Collins G, Berentsen S, Braun BS, Zinter MS. Paroxysmal cold hemoglobinuria successfully treated with complement inhibition. Blood Adv. 2019 Nov 26;3(22):3575-3578. doi: 10.1182/bloodadvances.2019000897. PMID: 31738828; PMCID: PMC6880888.



