TL;DR
Fibrinolysis is the enzymatic reaction in dissolving fibrin clots after wound healing, restoring blood flow and preventing unwanted thrombus formation.
- Essential Players ▾:
- Plasminogen: The inactive precursor protein, waiting to be activated.
- Plasmin: The active enzyme responsible for cleaving fibrin and dismantling clots.
- Activators:
- tPA (tissue plasminogen activator): Primarily activates plasmin at the clot site.
- uPA (urokinase plasminogen activator): Regulates broader fibrinolysis in tissues and blood vessels.
- Inhibitors:
- Alpha2-antiplasmin: Quickly inactivates plasmin, preventing uncontrolled proteolysis.
- PAIs (plasminogen activator inhibitors): Regulate the activity of tPA and uPA.
- Pathways ▾:
- Intrinsic (uPA): uPA activates plasminogen directly or through other proteases, primarily in tissues and vasculature.
- Extrinsic (tPA): Tissue plasminogen activator (tPA) binds directly to fibrin and activates plasminogen, mainly at the site of a clot.
- Significance ▾:
- Maintains balance: Prevents excessive clotting (thrombosis) and bleeding.
- Wound healing: Dissolves clots after repair, allowing tissue regeneration.
- Medical applications: Fibrinolytic agents like tissue plasminogen activator (tPA) can dissolve clots in strokes and heart attacks.
Understanding fibrinolysis is crucial for diagnosing and treating various diseases:
- Bleeding disorders: Deficiencies in key players can lead to excessive bleeding.
- Thrombosis: Impaired fibrinolysis can worsen clot formation and increase risk of blood clots.
- DIC (disseminated intravascular coagulation): Can involve both excessive clot formation and breakdown.
*Click ▾ for more information
Hemostasis
Hemostasis ensures our blood flows effortlessly while also sealing leaks at the slightest injury. This intricate process balances clot formation (coagulation) and clot breakdown (fibrinolysis), maintaining a delicate equilibrium critical for both healthy blood flow and efficient wound healing.
Coagulation: Building the Plug
First, platelets like tiny bricks rush to the scene, adhering to the injury and each other, forming a temporary plug in primary hemostasis. Then, the coagulation cascade, a finely tuned sequence of enzymatic reactions, kicks in. This cascade orchestrates the conversion of fibrinogen into fibrin, a mesh-like web that reinforces the platelet plug, creating a sturdy clot. Calcium ions act as essential scaffolding, ensuring the fibrin mesh holds strong.
Fibrinolysis: The Cleanup Crew
Once the wound starts healing, another team, the fibrinolytic system, takes over. Plasminogen, another circulating protein, gets activated into plasmin, the demolition expert. Plasmin carefully snips apart the fibrin mesh, gradually dismantling the clot and restoring blood flow to the repaired tissue.
The Importance of Balance
This exquisite equilibrium between coagulation and fibrinolysis is vital.
If the brakes on coagulation fail, excessive clot formation (thrombosis) can occur within intact blood vessels, blocking vital arteries and veins. This can lead to potentially life-threatening conditions like deep vein thrombosis, pulmonary embolism, and stroke.
Conversely, if fibrinolysis goes into overdrive, the clot dissolves too quickly, leading to uncontrolled bleeding and delayed wound healing. This can pose significant risks in conditions like hemophilia and traumatic injuries.
Key Players of Fibrinolysis
Fibrinolysis plays a crucial role in dismantling clots after wound healing, maintaining healthy blood flow, and preventing unwanted thrombus formation.
Precursor and Active Enzyme
Plasminogen plays a pivotal role in fibrinolysis, acting as the inert precursor protein to the active enzyme plasmin (inactive zymogen).
Plasminogen circulates abundantly in the blood, accounting for 1-2% of total plasma protein. This ensures a readily available pool for plasmin generation whenever needed. Its specific structure includes five distinct domains, each with crucial functions.
Upon activation by specific enzymes called plasminogen activators, plasminogen undergoes a conformational change, exposing the active site of the resulting plasmin enzyme.
Plasminogen activation isn’t a haphazard process. It’s tightly regulated and primarily occurs at the site of a clot through specific mechanisms:
- Binding to fibrin: Plasminogen has a high affinity for fibrin within the clot, allowing it to be concentrated where its proteolytic activity is needed.
- Activator targeting: Specific plasminogen activators like tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA) bind to fibrin and plasminogen, facilitating their interaction and cleavage.
Once activated, plasmin becomes a potent serine protease, capable of cleaving fibrin at specific sites within its mesh-like structure. This targeted proteolysis gradually dismantles the clot, allowing blood flow to resume in the repaired tissue. The specificity of plasmin’s action minimizes damage to surrounding tissues and prevents uncontrolled proteolysis of other proteins in the blood.
Activators
- Tissue plasminogen activator (tPA): Plays a key role in localized fibrinolysis at the site of a clot by binding to fibrin within the clot, bringing plasminogen in close proximity and facilitating its activation by cleaving off a specific peptide bond. It is primarily used in thrombolytic therapy to dissolve occlusive clots in conditions like ischemic stroke and myocardial infarction.
- Urokinase plasminogen activator (uPA): Contributes to broader fibrinolysis regulation beyond the immediate clot area. It is primarily expressed in various tissues and at the blood vessel wall, activating plasminogen in the surrounding extracellular matrix and plays a role in tissue remodeling and inflammatory processes.
- Other activators
- Streptokinase: A bacterial protein with broader plasminogen activating activity used in the past, but largely replaced by tissue plasminogen activator (tPA) due to its higher risk of bleeding complications.
- Factor XIIa: An enzyme in the coagulation cascade can indirectly activate plasminogen under certain conditions.
Inhibitors
- Alpha2-antiplasmin (α2-AP): This is the primary physiological inhibitor of plasmin. It circulates in the blood at high concentrations and rapidly forms a stable, irreversible complex with plasmin, effectively inactivating it. This prevents widespread proteolysis of other proteins beyond the targeted fibrin degradation in a clot. Deficiencies in α2-AP can lead to increased bleeding risk.
- Plasminogen activator inhibitor (PAI): This family of proteins primarily regulates the activity of the plasminogen activators, tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA), preventing their uncontrolled activation and subsequent plasmin generation. Elevated PAI levels can lead to impaired fibrinolysis and increased thrombosis risk. Two main subtypes exist:
- PAI-1: Specifically targets tissue plasminogen activator (tPA), and is the major regulator of fibrinolysis in the circulation.
- PAI-2: Primarily regulates urokinase plasminogen activator (uPA) activity in tissues and at the blood vessel wall.
Other Molecules
- Fibrin: The substrate for plasmin, forming the mesh-like structure of the clot.
- Calcium ions: Important for stabilizing the fibrin mesh and regulating plasmin activity.
- Annexin A2: A protein that binds to phosphatidylserine exposed on activated platelets and aids in plasminogen binding and clot lysis.
- Tissue inhibitors of metalloproteinases (TIMPs): Indirectly contribute to fibrinolysis by inhibiting enzymes that can degrade plasminogen activators.
The Process of Fibrinolysis
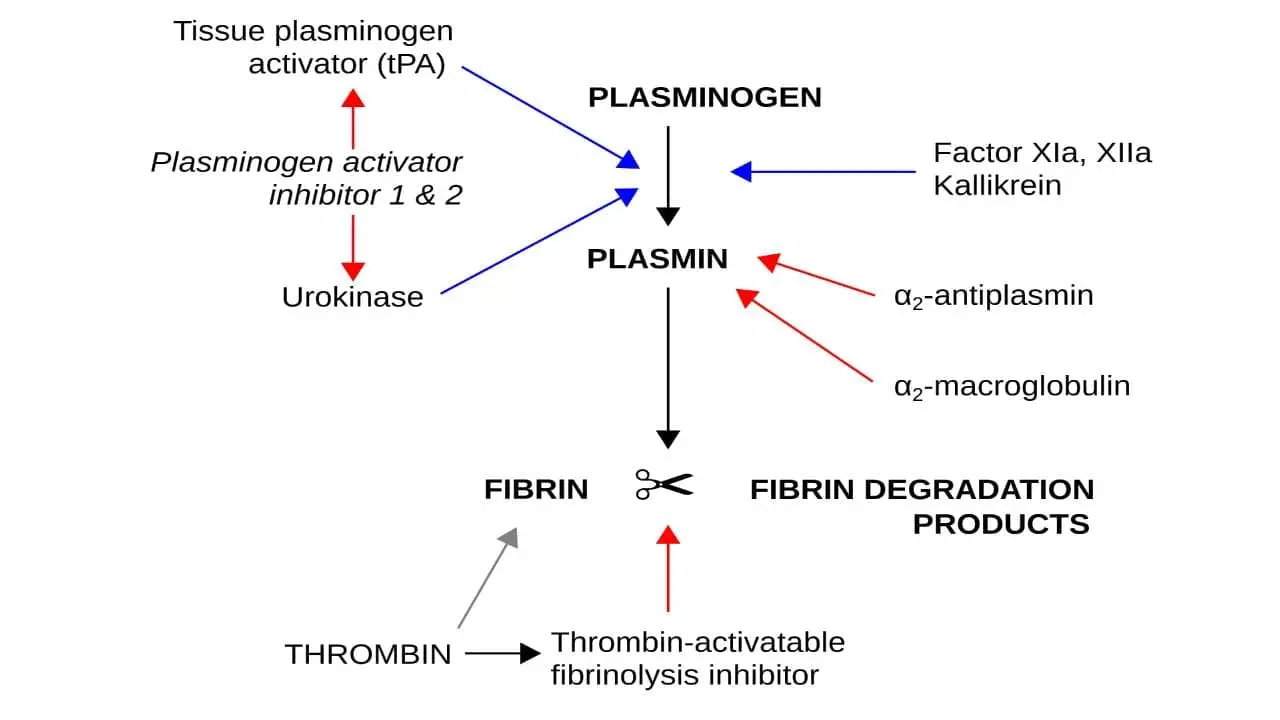
Plasminogen, the key to clot breakdown, sits in the wings, waiting for the right cue. This cue comes in the form of two distinct activation pathways: the intrinsic (urokinase plasminogen activator (uPA)) and the extrinsic (tissue plasminogen activator (tPA)).
Intrinsic Pathway (uPA/uPAR System)
This pathway operates primarily within tissues and at the blood vessel wall. The urokinase plasminogen activator (uPA) is primarily expressed in tissues and at the blood vessel wall.
Urokinase plasminogen activator (uPA) binds to a specific receptor on the cell surface called uPAR (urokinase plasminogen activator receptor). Urokinase plasminogen activator (uPA) forms complexes with its specific inhibitor, PAI-1, keeping it in check.
Tissue damage or inflammation disrupts this complex, freeing urokinase plasminogen activator (uPA). Once bound, urokinase plasminogen activator (uPA) undergoes a conformational change, increasing its affinity for plasminogen.
Urokinase plasminogen activator (uPA) then cleaves a specific peptide bond in plasminogen, transforming it into the active enzyme, plasmin.
The uPA/uPAR system ensures localized fibrinolysis, confining plasmin activity to the immediate vicinity of the clot. This minimizes damage to surrounding tissues and prevents unwanted systemic activation.
The newly formed plasmin further activates urokinase plasminogen activator (uPA) in a positive feedback loop, amplifying the localized clot breakdown.
Extrinsic Pathway (tissue plasminogen activator (tPA)/Fibrin System)
This systemic pathway features tissue plasminogen activator (tPA), primarily circulating in the blood. Produced by endothelial cells lining blood vessels, tissue plasminogen activator (tPA) circulates in the blood in an inactive form.
Upon encountering fibrin within a clot, tissue plasminogen activator (tPA) undergoes a conformational change, activating its plasminogen-cleaving ability. Tissue plasminogen activator (tPA) preferentially activates plasminogen bound to fibrin, further restricting its activity to the clot area.
Unlike uPA, tissue plasminogen activator (tPA) cleaves plasminogen more efficiently, but its systemic presence requires tighter control.
Fibrin dismantling
Once activated, plasmin gets to work dismantling the fibrin mesh. It does this by meticulously cleaving specific peptide bonds within the fibrin molecule, effectively weakening its structure and causing the clot to unravel. This targeted action minimizes damage to surrounding proteins and tissues.
Localization Matters
Both pathways emphasize the importance of localized fibrinolysis. This ensures that clot breakdown occurs only where needed, preventing excessive bleeding and maintaining the delicate balance between hemostasis and fibrinolysis. Tight regulation by inhibitors and clearance mechanisms further ensures plasmin’s activity is kept in check.
Disordered Fibrinolysis
Disordered fibrinolysis can lead to either excessive bleeding or an increased risk of thrombosis (clotting). The causes are generally categorized as inherited or acquired, and a range of specialized tests is required for diagnosis.
Causes of Disordered Fibrinolysis
Disordered fibrinolysis can occur due to deficiencies or excesses of the proteins that regulate the breakdown of blood clots.
Excessive Fibrinolysis (Hyperfibrinolysis)
This leads to premature breakdown of clots and results in bleeding.
- Inherited Causes: These are rare and typically involve a deficiency in a key fibrinolysis inhibitor.
- α2-Antiplasmin (α2-AP) Deficiency: A rare autosomal recessive condition where the primary inhibitor of plasmin is missing, leading to uncontrolled clot breakdown.
- Plasminogen Activator Inhibitor-1 (PAI-1) Deficiency: A deficiency in PAI-1, which normally inhibits plasminogen activators, leading to enhanced fibrinolysis.
- Quebec Platelet Disorder (QPD): A unique disorder where platelets contain an excess of urokinase-type plasminogen activator (uPA), which is released and causes premature clot lysis.
- Acquired Causes: These are more common and are often secondary to other medical conditions.
- Trauma: Severe trauma with hypoperfusion can cause an overwhelming activation of the fibrinolytic system.
- Liver Disease: The liver produces most of the proteins involved in fibrinolysis, so severe liver disease (e.g., cirrhosis) can lead to a state of hyperfibrinolysis.
- Disseminated Intravascular Coagulation (DIC): A complex condition that can lead to both widespread clotting and subsequent excessive bleeding due to the consumption of clotting factors and activation of fibrinolysis.
- Certain Cancers: Some types of cancer, like acute promyelocytic leukemia, can induce hyperfibrinolysis.
Hypofibrinolysis
Hypofibrinolysis is a condition of impaired or reduced fibrinolysis, which means the body is less efficient at breaking down blood clots. This leads to a persistent state of clotting and increases the risk of thrombotic events like deep vein thrombosis (DVT), pulmonary embolism (PE), and myocardial infarction.
The causes of hypofibrinolysis can be both inherited and acquired.
- Inherited Causes: These are genetic conditions that affect the proteins involved in fibrinolysis, making the system less active.
- Plasminogen Activator Inhibitor-1 (PAI-1) Excess: This is the most common inherited cause of hypofibrinolysis. A genetic polymorphism in the PAI-1 gene (4G/4G) leads to a higher production of PAI-1, which is the primary inhibitor of the clot-dissolving enzyme activators. This results in an impaired ability to break down clots and is linked to an increased risk of venous thrombosis and even recurrent miscarriages.
- Elevated Lipoprotein(a) [Lp(a)]: High levels of Lp(a) can interfere with fibrinolysis. Lp(a) is structurally similar to plasminogen, and it can compete for binding sites on the fibrin clot, preventing plasminogen from binding and being activated to plasmin. This reduces the overall rate of clot dissolution.
- Acquired Causes: These are more common and are often secondary to other medical conditions or lifestyle factors.
- Elevated PAI-1: Besides the genetic cause, elevated PAI-1 levels can be acquired. It is often associated with conditions like obesity, insulin resistance, type 2 diabetes, and metabolic syndrome. Adipose tissue is a primary source of PAI-1, and its overproduction in these conditions significantly impairs fibrinolysis.
- Dyslipidemia: High levels of triglycerides and cholesterol can impair fibrinolysis. Studies have shown a correlation between elevated serum triglycerides and increased PAI-1 levels, linking lipid metabolism with an impaired ability to clear clots.
- Chronic Inflammation: Conditions that cause chronic inflammation, such as autoimmune diseases, can lead to elevated PAI-1 and other antifibrinolytic factors.
- Aging: The fibrinolytic system naturally becomes less efficient with age. This is often accompanied by an age-related increase in plasma PAI-1 levels, contributing to the higher incidence of thrombotic cardiovascular diseases in the elderly.
Investigations for Disordered Fibrinolysis
Diagnosing disordered fibrinolysis can be challenging as routine coagulation tests (like prothrombin time or APTT) are often normal. A high index of suspicion is required, and specialized tests are used.
- Global Assays: These tests assess the overall function of the fibrinolytic system.
- Euglobulin Clot Lysis Time (ECLT): A traditional test that measures the time it takes for a clot to break down. A shortened time suggests hyperfibrinolysis.
- Thromboelastography (TEG) and Rotational Thromboelastometry (ROTEM): These point-of-care tests can provide a visual representation of clot formation and breakdown in real-time, helping to identify hyperfibrinolysis.
- Specific Protein Assays: These are used to measure the levels and function of individual fibrinolytic proteins.
- α2-Antiplasmin Activity and Antigen Levels: Used to diagnose α2-AP deficiency.
- PAI-1 Activity and Antigen Levels: Used to diagnose PAI-1 deficiency.
- Plasminogen Activator (tPA/uPA) Assays: Can be used to measure the levels of plasminogen activators, which may be elevated in some disorders.
- Fibrin Degradation Products (FDPs) and D-dimer: These tests measure the byproducts of fibrin breakdown. While not specific to fibrinolysis disorders, elevated levels indicate that a significant amount of clotting and subsequent breakdown is occurring, which is seen in conditions like DIC.
- Genetic Testing: Recommended for patients with a high suspicion of an inherited disorder to identify the specific gene mutation responsible.
Treatment and Management of Disordered Fibrinolysis
Disordered fibrinolysis requires different treatment strategies depending on whether it leads to excessive bleeding (hyperfibrinolysis) or an increased risk of clotting (hypofibrinolysis). The primary goal is to restore the hemostatic balance.
Management of Hyperfibrinolysis
Hyperfibrinolysis is characterized by the premature breakdown of blood clots, which can lead to significant and even life-threatening bleeding. The treatment focuses on inhibiting the overactive fibrinolytic system.
- Antifibrinolytic Agents: These are the cornerstone of treatment. They work by inhibiting the activation of plasminogen to plasmin, the enzyme responsible for breaking down clots.
- Tranexamic Acid (TXA): This is a synthetic derivative of the amino acid lysine. It binds to plasminogen and plasmin, preventing them from attaching to the fibrin clot and breaking it down. TXA is widely used in trauma, surgery, postpartum hemorrhage, and other bleeding conditions where hyperfibrinolysis is a factor.
- Epsilon-Aminocaproic Acid (EACA): Similar to TXA, EACA is a lysine analog that inhibits the fibrinolytic system. It is used in situations like post-surgical bleeding and to manage bleeding in certain inherited disorders.
- Supportive Care: In cases of acute, severe bleeding, supportive measures are crucial.
- Blood Product Transfusions: Patients may require transfusions of packed red blood cells to treat anemia, fresh frozen plasma to replace clotting factors, and platelets to address thrombocytopenia.
- Management of the Underlying Cause: When hyperfibrinolysis is secondary to a condition like severe trauma, liver disease, or disseminated intravascular coagulation (DIC), the primary focus is on treating the underlying illness to normalize the fibrinolytic system.
Management of Hypofibrinolysis
Hypofibrinolysis is a state of impaired clot breakdown, which leads to an increased risk of thrombosis. Management strategies focus on reducing this thrombotic risk and, in an emergency, actively dissolving existing clots.
- Anticoagulant Therapy: For patients with hypofibrinolysis and a history of thrombosis, long-term anticoagulant therapy is often prescribed to prevent future clots.
- Direct Oral Anticoagulants (DOACs): These are commonly used to inhibit specific clotting factors.
- Warfarin: This medication prevents the formation of vitamin K-dependent clotting factors.
- Heparin: Used for both short-term and long-term management, especially in hospital settings.
- Thrombolytic Therapy: In acute, life-threatening thrombotic events such as a heart attack, stroke, or pulmonary embolism, powerful clot-dissolving medications are used.
- tPA (Tissue Plasminogen Activator): Drugs like Alteplase, Reteplase, and Tenecteplase are recombinant forms of tPA. They are administered to rapidly activate plasminogen and break down a new clot. This is an emergency treatment with a narrow time window.
- Addressing Underlying Acquired Causes: Since hypofibrinolysis is often linked to conditions like diabetes, metabolic syndrome, and obesity, managing these comorbidities is a critical part of the long-term management. Lifestyle changes, including diet and exercise, can help normalize PAI-1 levels and improve fibrinolytic potential.
Frequently Asked Questions (FAQs)
What is the difference between fibrinolysis and thrombolysis?
Fibrinolysis is the natural process by which the body breaks down blood clots. It’s a normal physiological process that occurs to prevent clots from growing too large or blocking blood vessels.
Thrombolysis is the medical intervention to dissolve blood clots. It’s a therapeutic process that uses drugs like tPA to accelerate fibrinolysis and break down clots that are causing problems, such as those in a heart attack or stroke.
Essentially, fibrinolysis is the body’s natural clot-busting system, while thrombolysis is the medical treatment to enhance or mimic that process.
What is fibrinolysis for STEMI?
Fibrinolysis is a crucial treatment for STEMI (ST-segment elevation myocardial infarction), which is a heart attack caused by a complete blockage of a coronary artery.
- How it works: Fibrinolytic drugs, like tPA, break down the blood clot blocking the coronary artery, restoring blood flow to the heart muscle.
- Time is critical: To be most effective, fibrinolysis should be administered within the first few hours of symptom onset.
- Benefits: It can significantly reduce heart damage and improve survival rates when given promptly.
Challenges and Considerations
While fibrinolysis is a lifesaving treatment, it’s important to note:
- Risk of bleeding: These drugs can increase the risk of bleeding. Patients are carefully monitored for this complication.
- Alternative treatment: Primary percutaneous coronary intervention (PCI) is generally preferred over fibrinolysis when available, as it offers better outcomes. However, fibrinolysis remains a valuable option in areas without immediate access to PCI.
What are the two types of fibrinolysis?
There are two main types of fibrinolysis:
- Primary fibrinolysis: This is the natural process your body uses to dissolve blood clots after they’ve served their purpose. It’s a controlled process that prevents excessive clot formation.
- Secondary fibrinolysis: This occurs due to an external factor, such as:
- Medication (like tPA)
- A medical condition (like disseminated intravascular coagulation or DIC)
- Other causes (like severe infections)
Secondary fibrinolysis can sometimes be excessive, leading to bleeding problems.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Saba HI, Roberts HR. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management (Wiley Blackwell). 2014.
- DeLoughery TG. Hemostasis and Thrombosis 4th Edition (Springer). 2019.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.
- Kaushansky K, Levi M. Williams Hematology Hemostasis and Thrombosis (McGraw-Hill). 2017.
- Mutch, N. J., & Medcalf, R. L. (2023). The fibrinolysis renaissance. Journal of thrombosis and haemostasis : JTH, 21(12), 3304–3316. https://doi.org/10.1016/j.jtha.2023.09.012
- Risman, R. A., Kirby, N. C., Bannish, B. E., Hudson, N. E., & Tutwiler, V. (2023). Fibrinolysis: an illustrated review. Research and practice in thrombosis and haemostasis, 7(2), 100081. https://doi.org/10.1016/j.rpth.2023.100081
- Bannish, B.E., Chernysh, I.N., Keener, J.P. et al. Molecular and Physical Mechanisms of Fibrinolysis and Thrombolysis from Mathematical Modeling and Experiments. Sci Rep 7, 6914 (2017). https://doi.org/10.1038/s41598-017-06383-w
- Samson, A. L., Alwis, I., Maclean, J. A. A., Priyananda, P., Hawkett, B., Schoenwaelder, S. M., & Jackson, S. P. (2017). Endogenous fibrinolysis facilitates clot retraction in vivo. Blood, 130(23), 2453–2462. https://doi.org/10.1182/blood-2017-06-789032
- Mosnier, L. O., & Bouma, B. N. (2006). Regulation of fibrinolysis by thrombin activatable fibrinolysis inhibitor, an unstable carboxypeptidase B that unites the pathways of coagulation and fibrinolysis. Arteriosclerosis, thrombosis, and vascular biology, 26(11), 2445–2453. https://doi.org/10.1161/01.ATV.0000244680.14653.9a