Procedure At-A-Glance
HPLC (High Performance Liquid Chromatography) Procedure (Specifically the VARIANT II Beta Thalassemia Short Program).
- Sample Preparation: Collect a blood sample, preferably in EDTA. Lysate is prepared from the blood sample according to the specific kit instructions.
- Sample Loading: The prepared sample is then automatically loaded onto the HPLC system’s column.
- Chromatographic Separation: The hemoglobin components within the sample are separated as they pass through the column, based on their differential interaction with the stationary and mobile phases.
- Detection: As the separated hemoglobin components elute from the column, they are detected by a spectrophotometer, which measures their absorbance at a specific wavelength.
- Data Analysis: The detected signals are then processed by the system’s software to generate a chromatogram, which displays peaks corresponding to different hemoglobin subtypes. The software quantifies each subtype’s percentage based on peak area.
- Interpretation ▾: The generated chromatogram and quantitative data are interpreted to identify and determine the relative proportions of various hemoglobin variants, aiding in the diagnosis of hemoglobinopathies.
*Click ▾ for more information
Introduction
HPLC, or High Performance Liquid Chromatography, is a powerful tool used to detect hemoglobinopathies. Hemoglobinopathies, a group of genetic disorders that affect the structure or production of hemoglobin, the oxygen-carrying protein in red blood cells, are among the most common single-gene disorders worldwide.
These disorders arise from mutations in the genes encoding hemoglobin subunits, leading to alterations in the protein’s structure, function, and stability. Hemoglobinopathies encompass a wide spectrum of conditions, ranging from mild anemia in asymptomatic cases to severe, life-threatening complications.
Hemoglobinopathies are broadly classified into two main categories: thalassemias and structural hemoglobin variants.
- Thalassemias: Thalassemias result from mutations in the genes encoding globin chains, leading to either reduced or absent production of specific globin chains. This imbalance in globin chain synthesis disrupts hemoglobin formation, resulting in anemia, a condition characterized by low red blood cell count or hemoglobin levels.
- Structural hemoglobin variants: Structural hemoglobin variants arise from mutations in the genes encoding globin chains, leading to alterations in the amino acid sequence of the protein. These alterations can affect hemoglobin’s structure, stability, and oxygen-binding affinity, leading to various clinical manifestations.
Among the most prevalent hemoglobinopathies are:
- Sickle cell disease (SCD): SCD is caused by a single mutation in the beta-globin gene, resulting in the production of abnormal hemoglobin S. Hemoglobin S, under low oxygen conditions, can polymerize within red blood cells, causing them to sickle and become rigid, leading to pain, fatigue, and other complications.
- Beta-thalassemia: Beta-thalassemia results from mutations in the beta-globin gene, leading to reduced or absent production of beta-globin chains. The severity of beta-thalassemia depends on the number of mutated genes inherited.
- Alpha-thalassemia: Alpha-thalassemia arises from mutations in the alpha-globin gene, leading to reduced or absent production of alpha-globin chains. Alpha-thalassemia can range from mild, asymptomatic cases to severe, transfusion-dependent anemia.
Diagnosis of hemoglobinopathies typically involves a combination of family history, clinical evaluation, and laboratory tests. Accurate identification of hemoglobin subtypes is essential for diagnosing and managing these conditions.
High performance liquid chromatography (HPLC) has emerged as a valuable tool for hemoglobin subtype identification due to its high sensitivity, specificity, and ability to separate and quantify various hemoglobin variants.
Principle of High Performance Liquid Chromatography (HPLC)
High performance liquid chromatography is a versatile and powerful analytical technique widely used in various scientific disciplines, including chemistry, biochemistry, pharmaceutical science, and environmental science. Its ability to separate and quantify complex mixtures of compounds with high sensitivity and resolution has made it an indispensable tool for a wide range of applications.
The Essence of HPLC: Separation and Detection
At the heart of high performance liquid chromatography lies the principle of separation based on the differential distribution of solutes between a stationary phase and a mobile phase. The stationary phase is typically a solid material packed inside a chromatographic column, while the mobile phase is a liquid solvent that flows through the column.
As the sample mixture is injected into the column, the solutes interact with the stationary phase and the mobile phase to varying degrees, causing them to travel through the column at different speeds. This separation is achieved based on the interplay of various factors, including the solute’s molecular size, polarity, and affinity for the stationary phase.
The Stationary Phase: The Scaffold for Separation
The stationary phase plays a crucial role in the separation process. It provides a surface for solutes to interact with and influences their distribution between the stationary and mobile phases.
Common stationary phases include silica gel, alumina, and bonded phases, which have specific functional groups attached to their surface to enhance selectivity for certain types of solutes.
The Mobile Phase: The Driving Force for Separation
The mobile phase, in the form of a liquid solvent, continuously flows through the chromatographic column, carrying the solutes along its path. The choice of solvent and its composition significantly impact the separation process. The solvent should be compatible with the stationary phase and the solutes to ensure proper interaction and separation.
Detection and Quantification
As solutes elute from the column, they are detected and quantified using a variety of detectors. Common detectors include UV-visible absorbance detectors, which measure the absorbance of light at specific wavelengths, and mass spectrometers, which identify and quantify solutes based on their mass-to-charge ratio.
HPLC Application Hemoglobin Subtype Identification
The ion exchange chromatography for hemoglobin separation relies on the selective interaction between charged groups on the silica-based exchange material (stationary phase) and the charged groups on the hemoglobin molecule (mobile phase).
The silica surface is modified with carboxyl groups, imparting a weak cationic charge, enabling the separation of hemoglobin (Hb) molecules based on their charge differences.
When a hemolysate containing a mixture of hemoglobins is introduced onto the resin, the elution rate of different hemoglobins is governed by the pH and ionic strength (ion concentration) of the buffered elution solution. The pH and ionic strength of the eluent are carefully controlled to achieve selective elution of different Hb variants.
Separation Mechanism
The separation of hemoglobin variants in high-performance liquid chromatography is primarily driven by two factors:
- Charge: Hemoglobin variants differ in their net charge due to variations in their amino acid sequences. The stationary phase can be designed to selectively interact with hemoglobin variants based on their charge, allowing for their separation.
- Hydrophobicity: Hemoglobin variants also exhibit differences in their hydrophobicity, the tendency of a molecule to repel or attract water. The stationary phase can be tailored to selectively interact with hemoglobin variants based on their hydrophobicity, further enhancing separation.
Detection and Quantification
As hemoglobin variants elute from the column, they are detected using a UV-visible absorbance detector. Hemoglobin absorbs light at specific wavelengths, and the intensity of the absorbed light is proportional to the concentration of the hemoglobin variant. This information is used to quantify the relative proportions of different hemoglobin subtypes in the sample.
Advantages of HPLC for Hemoglobin Subtype Identification
- High Sensitivity: High performance liquid chromatography (HPLC) can detect even small amounts of hemoglobin variants, making it suitable for analyzing samples with low hemoglobin concentrations.
- Specificity: High performance liquid chromatography (HPLC) can distinguish between closely related hemoglobin variants, providing accurate subtype identification.
- Quantification: High performance liquid chromatography (HPLC) allows for the quantification of different hemoglobin variants, providing insights into their relative proportions in the sample.
- Automation: High performance liquid chromatography (HPLC) methods can be automated, reducing manual labor and improving consistency.
VARIANTTM II Beta Thalassemia Short Program (Bio-Rad Laboratories Inc., Hercules, CA, USA)
The VARIANTTM II Beta Thalassemia Short Program employs the principles of ion-exchange high-performance liquid chromatography (HPLC) to accurately identify and quantify hemoglobin variants. This automated method utilizes the VARIANTTM II Sampling Station (VSS) for sample preparation and injection and the VARIANTTM Chromatographic Station (VCS) for separation and detection.
Sample Preparation and Injection
The VSS automates the sample mixing, dilution, and injection process, ensuring precision and consistency. Prepared samples are then injected onto the analytical cartridge for separation and analysis.
Separation and Detection
The VCS employs dual pumps to deliver a precisely controlled gradient of increasing ionic strength to the analytical cartridge. This gradient facilitates the separation of HbA2 and HbF based on their differential ionic interactions with the cartridge material. The separated hemoglobin variants pass through a flow cell equipped with a filter photometer, which measures the absorbance changes at 415 nm. An additional filter at 690 nm compensates for background absorbance.
Data Analysis and Interpretation
The VARIANTTM II CDM (CDM) Software processes the raw data collected from each analysis. To aid in result interpretation, predefined windows have been established for commonly encountered hemoglobins based on their characteristic retention times. For each sample, the CDM generates a sample report and a chromatogram, providing detailed information on the eluted hemoglobin fractions, their retention times, peak areas, and fractional values.
Materials
- EDTA peripheral blood sample
- VARIANTTM Automated HPLC system
- Diluent buffer
- 2 phosphate buffers of different pH and ionic strengths
- HbA2/Hb F calibrator
- Lyphochek® Hemoglobin A2 Control, Bilevel (2 each of 2 levels)
Protocol
- To ensure the accuracy of the subsequent analyses, the HbA2/HbF Calibrator is analyzed at the start of each run to generate calibration factors for both hemoglobin A2 and F. These calibration factors will then be applied to calculate the area percentages for HbA2 and F in all subsequent analyses within the run. Additionally, the Lyphocheck Hemoglobin A2 Control (Levels 1 and 2 from Bio-Rad) is analyzed to verify that the concentration values of HbA2 and HbF remain within acceptable limits.
- A total of 5 uL EDTA blood sample is diluted with a 1 mL diluent buffer and followed by an analysis time of 6.5 minutes per sample.
- For individuals with low hemoglobin readings, they must be pre-diluted in the ratio of 1:100 wash buffer in order to fit the total area within 1,000,000 to 3,000,000 µvolt·second to be considered as valid results.
HPLC Interpretation
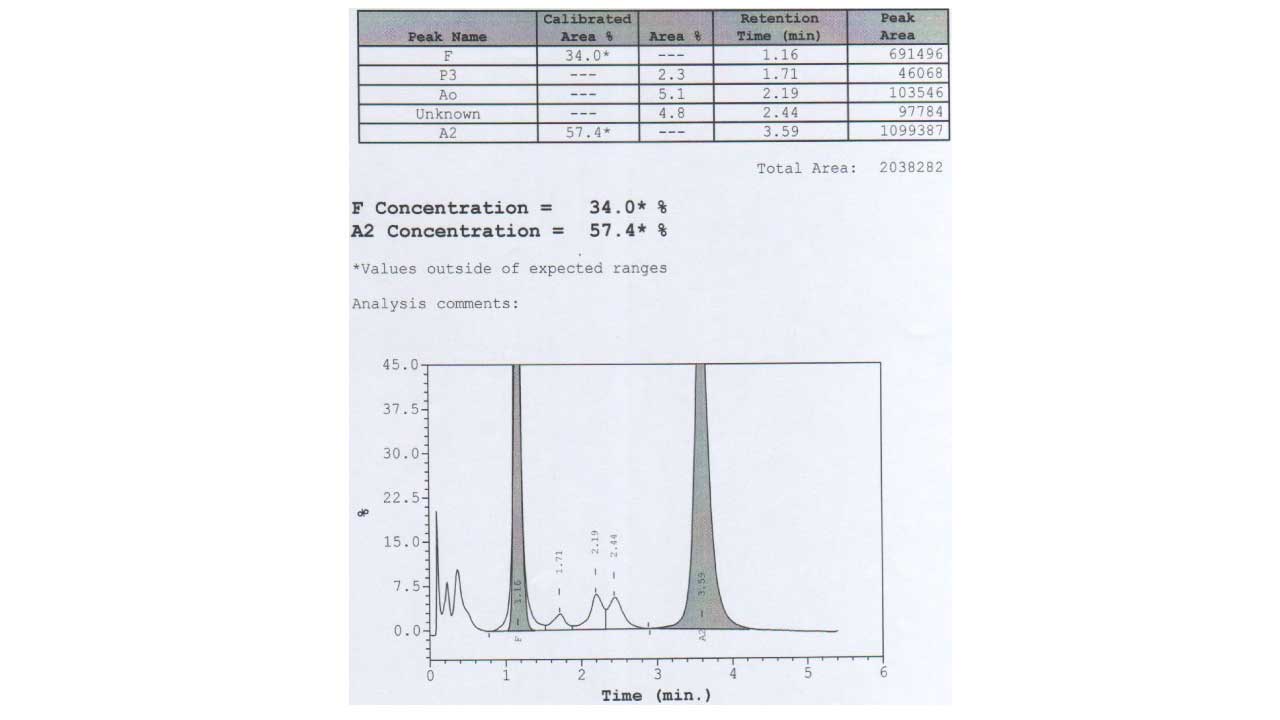
Peripheral whole blood can be effectively resolved into hemoglobin A, A2/E, F and other haemoglobins variants. This program has two calibrated areas (%), namely peak F and A2, whereby the HbF and HbA2 concentration can be measured.
Manufacturer assigned windows for Bio-Rad VARIANTTM HPLC System
| Peak Name | Window (minutes) | Retention Time (minutes) |
| F window | 1.00 – 1.30 | 1.15 |
| P2 window | 1.30 – 1.60 | 1.45 |
| P3 window | 1.60 – 1.90 | 1.75 |
| A0 window | 1.90 – 3.30 | 2.60 |
| A2 window | 3.30 – 3.90 | 3.60 |
| D window | 3.90 – 4.30 | 4.10 |
| S window | 4.30 – 4.70 | 4.50 |
| C window | 4.90 – 5.30 | 5.10 |
Common hemoglobin variants detected according to different time windows (retention times)
| Window / Peak Name | Retention Time (minutes) | Causes / Variant Hemoglobin |
| Pre-integration region | < 1 | Bilirubin, Hb H, Hb Barts |
| F window | 0.98 – 1.22 | HbF, Hb Okayama |
| P2 window | 1.28 – 1.50 | Glycated HbA, Hb Hope |
| P3 window | 1.50 – 1.90 | Aged samples, HbJ-Meerut, Hb J-Oxford |
| A window | 1.90 – 3.10 | HbA, Glycated HbS, intact Hb Koln |
| A2 window | 3.30 – 3.90 | HbE, Hb D-Iran, Hb Lepore, Hb G-Koushatta, Hb Zurich, Hb Korle Bu |
| D window | 3.90 – 4.30 | Hb D-Punjab, Hb G-Philadelphia |
| S window | 4.30 – 4.70 | HbS, Hb Q-Thailand, Hb Manitoba |
| C window | 4.90 – 5.30 | HbC, Hb Constant Spring, Hb Agenogi, Hb O-Arab |
Frequently Asked Questions (FAQs)
What are the normal percentages for different hemoglobin types (e.g., HbA1, HbA2, HbF) in adults?
In healthy adults, the normal percentages for the major hemoglobin types are generally as follows:
- Hemoglobin A (HbA): This is the predominant adult hemoglobin. Normal range is typically 95% to 98% of total hemoglobin.
- Hemoglobin A2 (HbA2): This is a minor adult hemoglobin. Normal range is typically 2% to 3% (some sources may state up to 3.5%).
- Hemoglobin F (HbF – Fetal Hemoglobin): In adults, HbF is present in very small amounts. Normal levels are generally less than 1% or 0.8% to 2%.
What factors can affect Hemoglobin HPLC test results?
Several factors can influence Hemoglobin High Performance Liquid Chromatography test results, potentially leading to inaccurate readings or difficulties in interpretation.
Presence of Hemoglobin Variants
- Common and Rare Hemoglobin Variants: The presence of other hemoglobin variants (e.g., HbS, HbC, HbE, HbD, HbG, HbO-Arab) can interfere with the accurate quantification of normal hemoglobins (HbA, HbA2, HbF) or even HbA1c, as they might co-elute or have similar retention times to the fractions being measured. HPLC systems are designed to resolve many common variants, but unusual or new variants might still pose challenges.
- Fetal Hemoglobin (HbF): Elevated levels of HbF, common in certain conditions (like beta-thalassemia major/intermedia, hereditary persistence of fetal hemoglobin), can interfere with the accurate measurement of other hemoglobin fractions.
Sample-Related Factors
- Age of Sample/Sample Degradation: Aged blood samples can show spurious peaks due to hemoglobin degradation or modification, which can interfere with the analysis.
- Sample Quality: Hemolysis (breakdown of red blood cells before analysis) can affect results.
- Transfusion: Recent blood transfusions (especially within the last 3-4 months) can significantly alter the patient’s hemoglobin profile, making it difficult to interpret endogenous hemoglobin levels.
- Iron Deficiency Anemia: Iron deficiency can sometimes lead to lower HbA2 values, potentially masking a beta-thalassemia trait or leading to misinterpretation.
- Other Anemias/Blood Disorders: Conditions like myelodysplastic syndromes, aplastic anemia, or even severe megaloblastic anemia can affect red blood cell production and turnover, indirectly influencing hemoglobin profiles.
Physiological Conditions:
- Pregnancy: Hormonal changes during pregnancy can affect blood parameters, though typically less directly on core hemoglobin variant percentages unless there’s an underlying hemoglobinopathy.
- Medications and Supplements: Certain drugs or supplements, though less common for direct interference with hemoglobin variant separation, can influence blood parameters or cause sample abnormalities. It’s always advisable to inform the healthcare provider about all medications being taken.
- Bilirubin and Other Substances: High levels of bilirubin or other compounds in the sample can sometimes appear as early eluting peaks or cause baseline disturbances.
Methodological/Technical Factors
- Instrumentation and Reagents: Differences in High Performance Liquid Chromatography instruments, column types, and reagent batches can lead to slight variations in retention times or peak resolution. Laboratories establish their own normal ranges and interpretation guidelines based on their specific equipment.
- Operator Technique: While automated, improper sample preparation or handling can introduce errors.
- Calibration and Quality Control: Inadequate calibration or poor quality control practices can lead to inaccurate results.
Can this test be used to screen family members for hemoglobin disorders?
High Performance Liquid Chromatography plays a vital role in both targeted family screening and broader population screening efforts to identify individuals at risk, provide genetic counseling, and prevent severe forms of inherited hemoglobin disorders.
Is this test done for newborns?
Yes, the Hemoglobin High Performance Liquid Chromatography test is very commonly done for newborns as part of newborn screening programs in many countries worldwide. High Performance Liquid Chromatography is a cornerstone technology in newborn screening for hemoglobinopathies, helping to give affected infants the best possible start to life through early diagnosis and management.
Early Detection of Serious Disorders: Hemoglobinopathies like sickle cell disease and thalassemia can lead to severe health complications, developmental delays, and even early mortality if left undiagnosed and untreated. Newborn screening allows for very early identification, often before symptoms even appear.
Prompt Intervention: Early diagnosis enables healthcare providers to implement timely interventions, such as prophylactic penicillin for sickle cell disease, appropriate vaccinations, and parental education. This significantly reduces morbidity and mortality rates.
High Prevalence: Hemoglobinopathies are among the most common inherited disorders globally. Screening at birth helps to identify affected infants in populations where these conditions are prevalent.
Favorable Sample Type: Newborn screening typically uses dried blood spot samples collected via a heel prick, which are suitable for High Performance Liquid Chromatography analysis. Cord blood samples can also be used.
Automation and Accuracy: Modern High Performance Liquid Chromatography systems are highly automated, rapid, sensitive, and specific, making them well-suited for high-volume newborn screening programs. While initial results are presumptive, they guide further confirmatory testing (often including molecular analysis) if an abnormality is detected.
What is HPLC best used for?
High-performance liquid chromatography (HPLC) excels at analyzing and separating a wide range of mixtures. Here are some of its most common applications:
Quality Control
- Pharmaceuticals: Ensuring the purity and consistency of drugs by identifying and measuring active ingredients and impurities across batches.
- Food and Beverage Industry: Verifying the quality and composition of food and drinks, detecting additives, preservatives, and potential contaminants.
Research Applications
- Drug Development: Identifying and characterizing new drug candidates, monitoring their breakdown products in the body.
- Biological Samples: Analyzing proteins, vitamins, and other biomolecules in blood, tissue samples, etc. This can aid in medical diagnosis and research.
- Environmental Analysis: Detecting pollutants like pesticides or herbicides in water and soil samples.
Other Applications
- Forensics: Identifying unknown substances in criminal investigations, like analyzing drug samples or explosives.
- Material Science: Characterizing polymers and other materials.
Is HPLC quantitative or qualitative?
High performance liquid chromatography (HPLC) can actually be used for both quantitative and qualitative analysis.
- Quantitative analysis: High performance liquid chromatography (HPLC) is best in measuring the amount of a specific component in a mixture. The detector in the high performance liquid chromatography (HPLC) system generates a signal based on the amount of analyte present. By comparing this signal to a calibration curve created using known standards, scientists can determine the concentration of the target compound in the sample.
- Qualitative analysis: While not its primary strength, high performance liquid chromatography (HPLC) can also be used to identify components in a mixture. This is often done by comparing the retention time (how long it takes a component to travel through the column) of an unknown peak to the retention time of a known standard compound. Additionally, some high performance liquid chromatography (HPLC) systems are coupled with detectors like mass spectrometry (MS) which provide more definitive identification based on the molecule’s mass-to-charge ratio.
High performance liquid chromatography’s (HPLC) strength lies in its versatility. It can handle complex mixtures, separate components based on subtle differences, and provide accurate quantitative data. This makes it a valuable tool across many scientific disciplines.
What are the advantages and disadvantages of High Performance Liquid Chromatography?
HPLC (High Performance Liquid Chromatography) boasts a range of advantages that make it a powerful analytical tool, but it also has limitations to consider.
Advantages
- High Separation Power: High performance liquid chromatography (HPLC) excels at separating complex mixtures into their individual components due to the strong interaction between the stationary phase and the sample. This allows for highly resolved peaks in the chromatogram, making it easier to identify and quantify individual components.
- Sensitivity: Modern high performance liquid chromatography (HPLC) detectors are incredibly sensitive, allowing for detection of very low concentrations of analytes in a sample. This is crucial for applications like drug metabolite analysis or environmental pollutant detection.
- Versatility: High performance liquid chromatography (HPLC) can be applied to a wide range of samples as long as the components are soluble in a liquid solvent. This makes it a valuable tool across various scientific disciplines.
- Quantification: High performance liquid chromatography (HPLC) can be used to accurately measure the amount of a specific component in a mixture. This is achieved by comparing the detector signal of the unknown sample to a calibration curve obtained with known standards.
- Automation: Modern high performance liquid chromatography (HPLC) systems are highly automated, reducing human error and increasing reproducibility of results.
Disadvantages
- Cost: High performance liquid chromatography (HPLC) instruments are expensive to purchase and maintain. The solvents and columns used can also be costly.
- Complexity: Operating and optimizing high-performance liquid chromatography (HPLC) methods can be complex, requiring trained personnel. Troubleshooting issues can also be time-consuming.
- Sample Preparation: High performance liquid chromatography (HPLC) often requires extensive sample preparation steps, such as filtration and dilution, which can be time-consuming and may lead to sample loss.
- Limited Applicability to Non-Soluble Samples: Compounds that are not soluble in a suitable liquid solvent cannot be analyzed using high performance liquid chromatography (HPLC).
- Time Consumption: While faster than traditional chromatography methods, high performance liquid chromatography (HPLC) analysis times can still vary depending on the complexity of the sample and the desired separation.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- George E, Jamal AR, Khalid F, Osman KA. High performance liquid chromatography (HPLC) as a screening tool for classical Beta-thalassaemia trait in malaysia. Malays J Med Sci. 2001 Jul;8(2):40-6. PMID: 22893759; PMCID: PMC3413648.
- PK Gupta, H Kumar, S Kumar, M Jaiprakash. Cation Exchange High Performance Liquid Chromatography for Diagnosis of Haemoglobinopathies. Medical Journal Armed Forces India, 2009;65(1):33-37. https://doi.org/10.1016/S0377-1237(09)80051-8.
- M Dogaru, D Coriu, T Higgins. Comparison of two analytical methods (electrophoresis and HPLC) to detect thalassemias and hemoglobinopathies. Revista Română de Medicină de Laborator 2007; 9(4):39-48.
- Khera, R., Singh, T., Khuana, N., Gupta, N., & Dubey, A. P. (2015). HPLC in characterization of hemoglobin profile in thalassemia syndromes and hemoglobinopathies: a clinicohematological correlation. Indian journal of hematology & blood transfusion : an official journal of Indian Society of Hematology and Blood Transfusion, 31(1), 110–115. https://doi.org/10.1007/s12288-014-0409-x
- Baig, Mirza A.; Swamy, KB1; Baksh, Ameen D.2; Bahashwan, Ahmed3; Moshrif, Yasser4; Al Sawat, Abdullah5; Al Mutairi, Nabeel6; Alharbi, Nader7. Evaluation of role of HPLC (Merits & Pitfalls), in the diagnosis of various hemoglobinopathies & thalassemic syndromes. Indian Journal of Pathology and Microbiology 64(3):p 518-523, Jul–Sep 2021. | DOI: 10.4103/IJPM.IJPM_709_20
- Periyavan S., et al. HPLC first approach in detecting thalassemia and other common hemoglobinopathies is more cost and time effective. Front. Hematol., 17 March 2025. Sec. Red Cells, Iron and Erythropoiesis. Volume 4 – 2025. https://doi.org/10.3389/frhem.2025.1461498
- Ou, C. N., & Rognerud, C. L. (2001). Diagnosis of hemoglobinopathies: electrophoresis vs. HPLC. Clinica chimica acta; international journal of clinical chemistry, 313(1-2), 187–194. https://doi.org/10.1016/s0009-8981(01)00672-6
- Joshi A, Gohil S, Saxena B, Jatale R, Chadha K, Haryan R, et al. Hemoglobinopathies by HPLC: A 3 Year Study of 106,277 Cases. Ann of Pathol and Lab Med [Internet]. 2022 Jul. 13 [cited 2025 Jun. 30];9(6):A126-131