This blog post provides an overview of Chronic Myeloid Leukemia (CML) and its treatment strategies based on the most recent guideline (2025) from the National Comprehensive Cancer Network (NCCN).
TL;DR
- Tyrosine kinase inhibitors (TKIs) are the primary treatment for CML, targeting the abnormal protein produced by the Philadelphia chromosome.
- Other treatment options include chemotherapy and hematopoietic stem cell transplant (HSCT), mainly used in advanced phases or if TKIs are ineffective.
- Regular monitoring of treatment response using tests like qPCR to measure BCR::ABL1 levels is crucial.
- While TKIs are generally effective, they can have side effects, and some drugs and supplements may interfere with their efficacy.
What is Chronic Myeloid Leukemia (CML)?
CML is a type of cancer that starts in the bone marrow, specifically in cells called myeloid cells. Myeloid cells are immature cells that the body uses to create different mature blood cells: red blood cells (which carry oxygen), white blood cells (which fight infection), and platelets (which help with clotting).
CML is a relatively rare hematologic malignancy. The annual incidence is estimated to be around 1 to 2 cases per 100,000 adults. CML is primarily a disease of adults, with a median age at diagnosis of around 60 years. However, it can occur at any age, including in children and adolescents, though less commonly.
What causes Chronic Myeloid Leukemia (CML)?
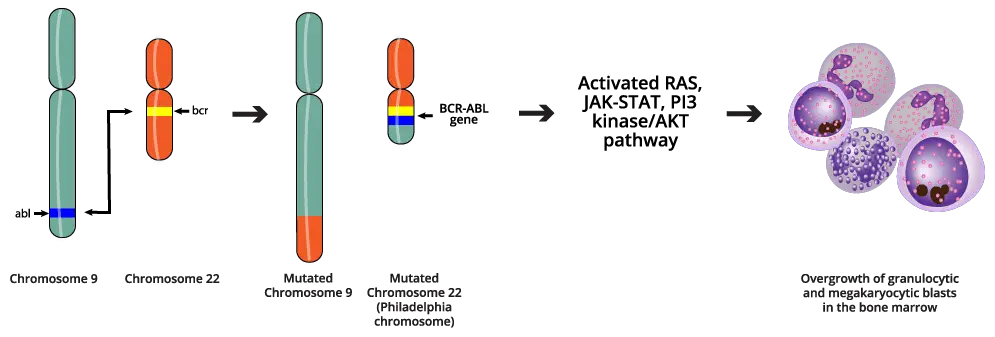
The hallmark of CML is the presence of the Philadelphia chromosome, resulting from a reciprocal translocation between the long arms of chromosomes 9 and 22. This is a specific genetic abnormality in the bone marrow’s hematopoietic stem cells. CML arises from an acquired (not inherited) genetic mutation in a hematopoietic stem cell. This means the change occurs during a person’s lifetime and is not passed down from parent to child.
The translocation results in the fusion of two genes: the BCR (breakpoint cluster region) gene from chromosome 22 and the ABL1 (Abelson murine leukemia viral oncogene homolog 1) gene from chromosome 9. This specific abnormality is known as the Philadelphia chromosome (Ph chromosome) which also can be written as t(9;22)(q34;q11). The swapping of chromosome pieces results in the formation of a new, abnormal gene called BCR::ABL1.
The BCR::ABL1 gene produces an abnormal protein called a tyrosine kinase. This BCR::ABL1 tyrosine kinase is constitutively active, meaning it’s always “turned on.” The overactive BCR::ABL1 tyrosine kinase drives excessive and unregulated proliferation of myeloid cells. These cells grow uncontrollably and don’t function properly, spilling into the bloodstream.
What are the signs and symptoms of Chronic Myeloid Leukemia (CML)?
In its early stages, many people with CML may not have any symptoms. When symptoms do appear, they can be vague and easily mistaken for those of other illnesses.
Common signs and symptoms of CML can include:
- Fatigue: Feeling unusually tired or weak.
- Weakness: A general lack of strength.
- Shortness of breath: Difficulty breathing, especially during physical activity.
- Night sweats: Heavy sweating during sleep.
- Weight loss: Losing weight without trying.
- Fever: Having a higher-than-normal body temperature.
- Bone pain: Aching in the bones.
- Enlarged spleen: This can cause a feeling of fullness or pain in the left side of the abdomen below the ribs.
- Frequent infections: Increased susceptibility to infections.
- Bleeding easily: Unusual bruising or nosebleeds.
What are the phases of Chronic Myeloid Leukemia (CML)?
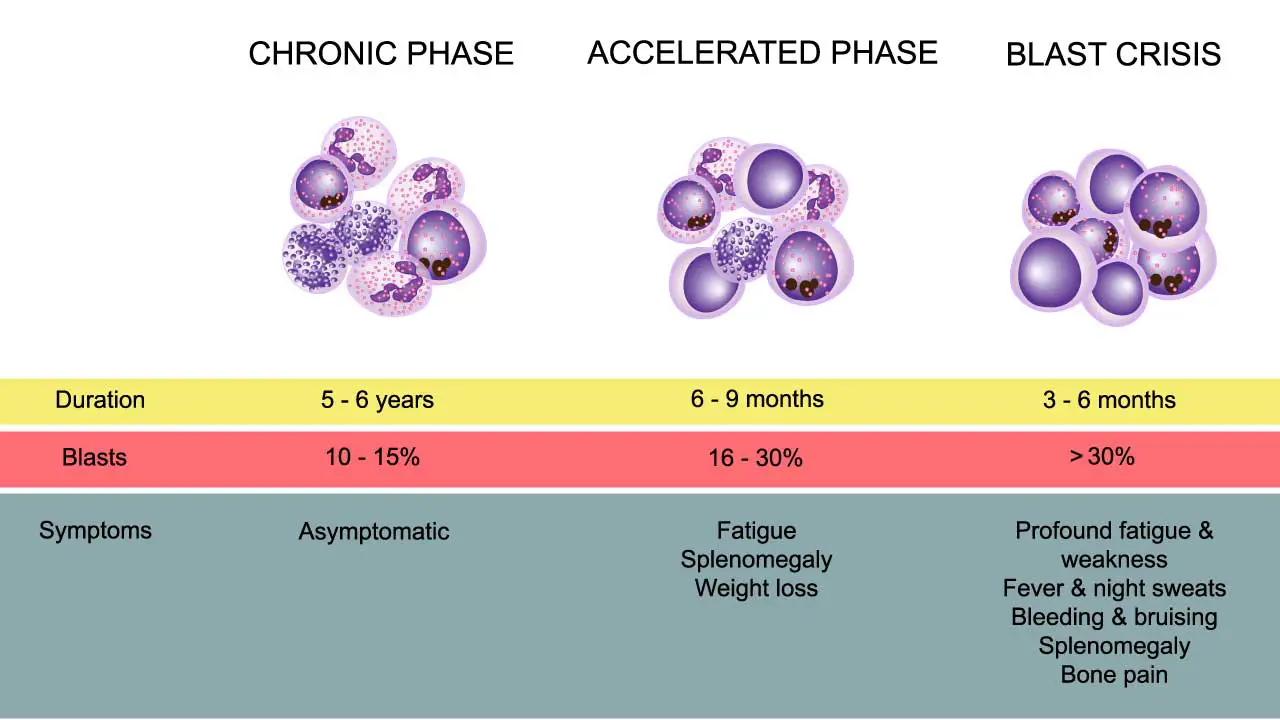
Chronic myeloid leukemia (CML) typically progresses through three distinct phases. These phases are characterized by the proportion of immature white blood cells (blast cells) in the blood and bone marrow, as well as the severity of symptoms and response to treatment. It is crucial to accurately determine the phase of CML at diagnosis, as this significantly influences treatment decisions and prognosis.
Chronic Phase
This is the earliest and most common phase at diagnosis. It is characterized by a relatively low number of blast cells in the blood and bone marrow (less than 10%). Patients in the chronic phase may have few or no symptoms, or they may experience mild symptoms such as fatigue, weight loss, or an enlarged spleen.
The chronic phase generally responds well to standard treatment with tyrosine kinase inhibitors (TKIs). The goal of treatment in this phase is to achieve and maintain a stable chronic phase and prevent progression to more advanced phases.
Accelerated Phase
This is a transitional phase where the disease is progressing. It is defined by an increase in blast cells (10-19%) in the blood or bone marrow, or other signs of disease progression. Symptoms may worsen and can include fever, bone pain, enlarged spleen, and fatigue.
The accelerated phase is more difficult to treat than the chronic phase, and there is a higher risk of progression to blast crisis.
Blast Crisis
This is the most advanced and aggressive phase of CML. It is characterized by a high number of blast cells (20% or more) in the blood or bone marrow, similar to acute leukemia. Patients in blast crisis may experience severe symptoms, including infections, bleeding, and organ infiltration by leukemia cells.
Blast crisis is the most challenging phase to treat and has the poorest prognosis.
General Chronic Myeloid Leukemia (CML) Treatment
The primary goals of chronic myeloid leukemia treatment are to:
- Eliminate or significantly reduce the number of cells with the Philadelphia chromosome.
- Allow the bone marrow to function normally again.
- Prevent the disease from progressing to more advanced phases.
CML is treated with systemic therapy, a form of drug therapy that works throughout the body. Systemic therapy includes targeted therapy, immunotherapy, and chemotherapy. However, CML is most commonly treated with targeted therapy.
Targeted Therapy
This is the cornerstone of chronic myeloid leukemia treatment. Targeted therapy drugs, specifically tyrosine kinase inhibitors (TKIs), are designed to block the abnormal protein produced by the BCR::ABL1 gene. TKIs are taken orally (as pills) and are generally well-tolerated.
They have dramatically improved the long-term outcomes for most people with CML. Different TKIs are available, and the choice of which TKI to use depends on factors such as the phase of CML, patient-specific factors, and potential side effects.
TKIs that might be used as chronic myeloid leukemia treatment includes:
- Asciminib (Scemblix): It targets a different area of BCR::ABL1 and is used for chronic phase CML with the T315I mutation or as a third-line treatment for those without it. It should be avoided in people who have had pancreatitis.
- Bosutinib (Bosulif): This is a second-generation TKI. It may not be preferred for people with liver or gastrointestinal issues.
- Dasatinib (Sprycel): A second-generation TKI that may not be prescribed if you have lung disease or breathing issues. There is a lower-cost generic option.
- Imatinib (Gleevec): The first TKI approved as chronic myeloid leukemia treatment and still considered a good option, especially for older patients or those with low-risk chronic phase CML. A lower-cost generic version is available.
- Nilotinib (Tasigna): A second-generation TKI that may not be suitable for people with heart issues or electrolyte abnormalities. It can cause increased blood sugar, worsen peripheral vascular disease, and prolong the QT interval.
- Ponatinib (Iclusig): A third-generation TKI preferred for those with the T315I BCR::ABL1 gene mutation, but it can have serious side effects and is not typically used as a first-line therapy.
Common Side Effects of TKIs
- Anemia, neutropenia and thrombocytopenia
- Fatigue
- Musculoskeletal pain
- Nausea, diarrhea, and vomiting
- Skin changes, such as rash
- Fluid buildup in limbs (edema)
- Headaches and fevers
Severe Side Effects of TKIs
- Heart and liver issues
- Kidney failure
It is important to note that TKIs should not be taken during pregnancy or while breastfeeding, and patients should consult their care team before stopping any TKI.
Chemotherapy
Chemotherapy drugs kill rapidly dividing cells, including leukemia cells.
While TKIs are the primary chronic phase chronic myeloid leukemia treatment, chemotherapy may be used in the accelerated or blast phases to help bring the disease under control.
Chemotherapy can be given intravenously (into a vein) or orally.
Hematopoietic Stem Cell Transplant (HSCT)
Also known as bone marrow transplant, HSCT is a procedure to replace the patient’s diseased bone marrow with healthy stem cells. Stem cells are typically obtained from a matched donor (allogeneic HSCT).
HSCT can offer a potential cure for CML, but it also carries significant risks and potential complications.
Due to the effectiveness of TKIs, HSCT is now less commonly used as a first-line treatment and is generally reserved for patients who:
- Do not respond adequately to TKI therapy.
- Develop resistance to multiple TKIs.
- Are in the accelerated or blast phase.
It’s important to note that treatment decisions are individualized and depend on several factors, including the phase of CML, the patient’s overall health, and response to therapy.
Regular monitoring is crucial to assess treatment effectiveness and manage any side effects.
Treatment Strategies According to Phases
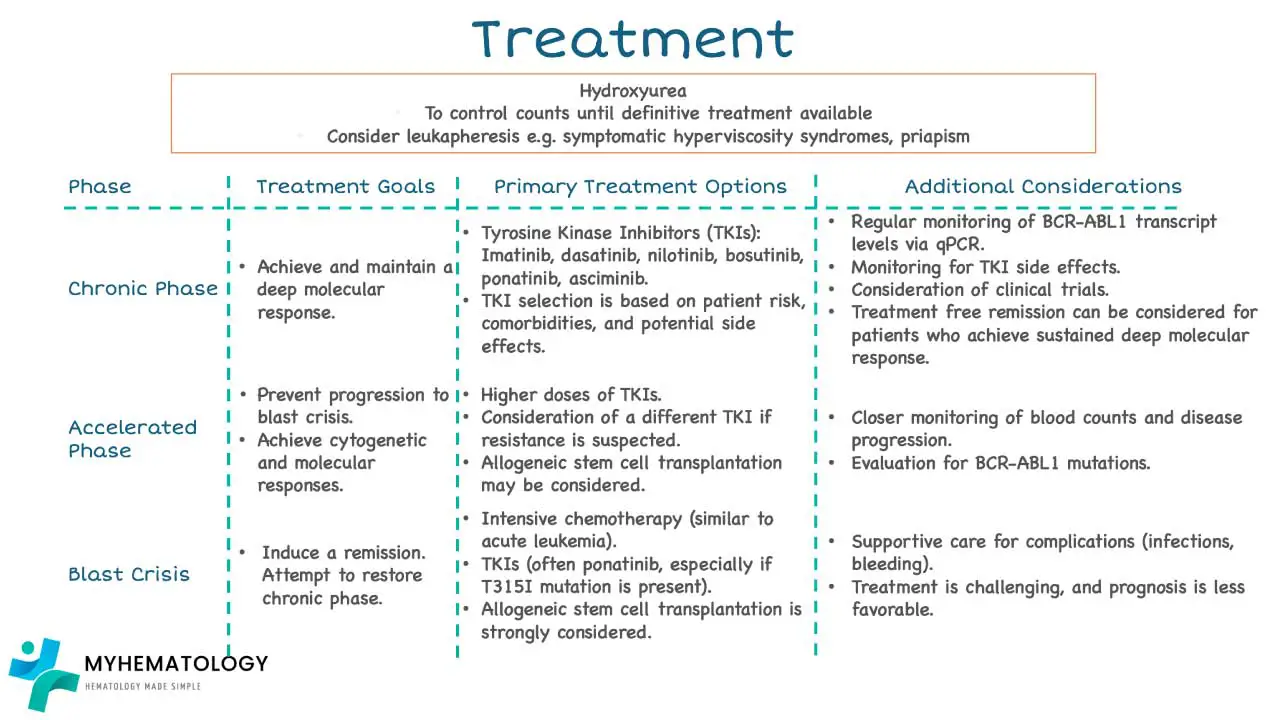
Chronic Phase Treatment
The primary chronic phase chronic myeloid leukemia treatment involves targeted therapy using tyrosine kinase inhibitors (TKIs). The choice of TKI depends on the patient’s risk group, potential side effects, drug interactions, and insurance coverage.
CML Scoring System
| Scoring System | Low Risk | Intermediate Risk | High Risk |
| Sokal Score | < 0.8 | 0.8 – 1.2 | > 1.2 |
| Hasford (EURO) Score | ≤ 780 | 781 – 1480 | > 1480 |
| EUTOS Score | ≤ 1.5680 | 1.5680 – 2.2185 | > 2.2185 |
- Low-risk CML: Preferred treatment options include Imatinib or generic Imatinib, second-generation TKIs (Bosutinib, Dasatinib, or Nilotinib), or a clinical trial.
- Intermediate or high-risk CML: The preferred treatment option is a second-generation TKI (Bosutinib, Dasatinib, or Nilotinib). Imatinib or generic Imatinib and clinical trials are also options.
Response to treatment is monitored using qPCR with the International Scale (IS) to measure BCR::ABL1 levels. Treatment goals include achieving specific response milestones, such as early molecular response (EMR), complete cytogenetic response (CCyR), major molecular response (MMR), and deep molecular response (DMR). If treatment is not effective, second-line treatment options are considered, which may involve switching to a different TKI.
| Treatment Milestones | Definition |
| Early molecular response (EMR) | When BCR::ABL1 is less than 10% at 3 months and 6 months. |
| Complete cytogenetic response (CCyR) | When tests don’t find any copies of the Philadelphia chromosome. Should be achieved within 12 months. |
| Major molecular response (MMR) | When BCR::ABL1 is less than 0.1%. |
| Deep molecular response (DMR) | Minimal or no copies of the abnormal BCR::ABL1 gene, or copies are detected at a very low level using a very sensitive test. When BCR::ABL1 is ≤ 0.01%. |
Advance Phase Treatment
The advanced phases of CML include the accelerated phase and the blast phase. These phases are more aggressive than the chronic phase and require more intensive treatment.
Accelerated Phase
The accelerated phase is a transitional phase between the chronic phase and the blast phase. It is characterized by an increase in blast cells (immature white blood cells) in the blood or bone marrow.
Criteria for Accelerated Phase
- Blood myeloblasts at 15 – 29%
- Total blood myeloblasts and promyelocytes ≥ 30%
- Blood basophils ≥ 20%
- Platelet count ≤ 100 x 109/L unrelated to therapy
- Additional mutation in Ph+ cells
- Increased lymphoblasts (suggesting possible blast phase)
Treatment Strategies for Accelerated Phase
The primary treatment goal in the accelerated phase is to bring the disease back under control and transition it to a chronic phase. Preferred treatment options include a clinical trial or second-generation TKIs (Bosutinib, Dasatinib, or Nilotinib) or Ponatinib. If mentioned TKIs are contraindicated then Imatinib can be useful or allosteric TKI (Asciminib).
Allogeneic hematopoietic stem cell transplantation should be considered if there is lack of response or there is disease progression.
The choice of treatment depends on individual patient factors, such as prior treatment history, response to therapy, and overall health status.
Blast Phase
The blast phase is the most advanced and aggressive stage of CML. It is characterized by a significant increase in blast cells (immature white blood cells) in the blood or bone marrow, defined as 20% or more.
Blast phase CML behaves similarly to acute leukemia and can be either myeloid (involving myeloid cells) or lymphoid (involving lymphoid cells). Patients in blast crisis often experience severe symptoms due to bone marrow failure and the infiltration of organs by blast cells.
Criteria for Blast Phase
The document specifies the following criteria for defining the blast phase:
- ≥20% blasts in the blood or bone marrow
Additionally, any increase in lymphoblasts is a concern that blast phase is beginning.
Treatment Strategies for Blast Phase
The treatment of blast phase CML is challenging and aims to achieve a remission (a decrease in the number of cancer cells) and transition the disease back to a chronic phase if possible.
In blast phase CML, the treatment approach is tailored to some extent based on whether the blast cells are myeloid (resembling acute myeloid leukemia – AML) or lymphoid (resembling acute lymphoblastic leukemia – ALL). This distinction is crucial because these two types of blast crisis respond differently to treatment.
Myeloid Blast Phase
- Chemotherapy: Intensive chemotherapy regimens similar to those used for AML are typically employed. The aim is to reduce the blast cell burden and restore chronic phase.
- Tyrosine Kinase Inhibitors (TKIs): TKIs are continued or adjusted, often in combination with chemotherapy, to target the CML clone. Ponatinib may be considered, especially if there’s a T315I mutation.
- Stem Cell Transplant: Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a critical option if the patient achieves a second chronic phase.
Lymphoid Blast Phase
- Chemotherapy: Chemotherapy regimens similar to those used for ALL are often used. These regimens typically include drugs like vincristine, prednisone, and others.
- Tyrosine Kinase Inhibitors (TKIs): TKIs are combined with the ALL-type chemotherapy to target the CML clone.
- Central Nervous System (CNS) Prophylaxis: Because lymphoid leukemia can spread to the CNS, preventive treatment (prophylaxis) to the brain and spinal cord may be given.
- Stem Cell Transplant: Allo-HSCT is considered if the patient responds to initial therapy.
The prognosis for patients in blast crisis is generally poor compared to those in the chronic phase, but treatment can sometimes be effective in achieving remission and allowing for further therapy, such as hematopoietic stem cell transplantation.
What drugs or supplements can interact with TKIs?
Some supplements that might interact with TKIs include:
- Turmeric
- Ginkgo biloba
- Green tea extract
- St. John’s Wort
- Antioxidants
Some medicines that might interact with TKIs include:
- Antacids
- Heart or blood pressure medicine
- Antidepressants
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022 Jul;36(7):1720-1748. doi: 10.1038/s41375-022-01620-2. Epub 2022 Jun 22. Erratum in: Leukemia. 2023 Sep;37(9):1944-1951. PMID: 35732829; PMCID: PMC9214472.
- https://www.nccn.org/patients/guidelines/content/PDF/cml-patient.pdf
- https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf
- Jabbour EJ, Sasaki K, Haddad FG, Issa GC, Garcia-Manero G, Kadia TM, Jain N, Yilmaz M, DiNardo CD, Patel KP, Kanagal-Shamanna R, Champlin R, Khouri IF, Dellasala S, Pierce SA, Kantarjian H. The outcomes of patients with chronic myeloid leukemia treated with third-line BCR::ABL1 tyrosine kinase inhibitors. Am J Hematol. 2023 Apr;98(4):658-665. doi: 10.1002/ajh.26852. Epub 2023 Jan 31. PMID: 36683287.
- https://www.lls.org/booklet/chronic-myeloid-leukemia