TL;DR
A Delayed Hemolytic Transfusion Reaction (DHTR) is an immune-mediated adverse event that occurs as a consequence of a blood transfusion. Its defining characteristic is the delayed onset, with signs and symptoms typically appearing 3 to 30 days post-transfusion.
- Pathophysiology ▾: Delayed hemolytic transfusion reaction occurs in a patient who has been previously sensitized to a foreign red blood cell antigen (from prior transfusion or pregnancy). The initial antibody response was too weak to be detected. Upon re-exposure, the immune system mounts a rapid and robust anamnestic (memory) response, producing a new or significantly increased titer of alloantibodies that bind to and destroy the transfused red blood cells.
- Signs and symptoms ▾: Fever, jaundice, dark urine, symptoms of anemia
- Laboratory investigation ▾:
- Clinical: Unexplained drop in hemoglobin and/or a mild fever.
- Laboratory Evidence of Hemolysis: Elevated indirect bilirubin and LDH, with decreased haptoglobin.
- Serological Evidence: A positive Direct Antiglobulin Test (DAT) and the identification of a newly appearing alloantibody in the patient’s serum that was not present in the pre-transfusion screen.
- Prevention ▾: The most effective prevention is to identify patients who have been previously sensitized through a thorough transfusion history and pre-transfusion antibody screening. All future transfusions for that patient must be with antigen-negative blood.
*Click ▾ for more information
Introduction
Blood transfusions are life-saving medical procedures, but they are not without risk. While immediate complications like acute hemolytic transfusion reaction (AHTR) are well-known, a less dramatic but clinically significant event is the delayed hemolytic transfusion reaction (DHTR).
Unlike acute hemolytic transfusion reaction (AHTR), which involves pre-existing antibodies, delayed hemolytic transfusion reaction is an anamnestic immune response that typically occurs days to weeks after a transfusion. This article provides a comprehensive overview of delayed hemolytic transfusion reaction, focusing on its pathophysiology, clinical features, diagnostic approach, and evidence-based management strategies.
Epidemiology and Incidence of Delayed Hemolytic Transfusion Reaction
The overall reported incidence of delayed hemolytic transfusion reaction is estimated to be approximately 1 in 2,500 to 1 in 11,000 transfusions. However, this is likely an underestimate. Many cases are mild, subclinical, or misdiagnosed due to the slow-onset nature of the symptoms, making it difficult to capture the true frequency. The patient’s hemoglobin may not drop significantly, or the symptoms may be mistaken for other post-transfusion conditions, such as simple fever or viral illness.
The most significant predisposing factor for delayed hemolytic transfusion reaction is a history of prior sensitization to red blood cell antigens. This can occur from a previous transfusion, pregnancy, or transplantation. The risk of developing a delayed hemolytic transfusion reaction is not uniform across all patient populations. Individuals who require frequent or chronic transfusions are at a significantly higher risk due to repeated exposure to foreign antigens. This includes patients with:
Additionally, multiparous women are at an increased risk due to exposure to fetal red blood cell antigens during pregnancy, a phenomenon that can lead to alloantibody formation.
Pathophysiology: The Anamnestic Immune Response
Delayed hemolytic transfusion reaction is primarily an extravascular hemolysis mediated by alloantibodies. The pathophysiology hinges on a fundamental immunological principle: the secondary, or anamnestic, immune response. It is a process that requires a prior exposure to a foreign red blood cell antigen, followed by a subsequent re-exposure.
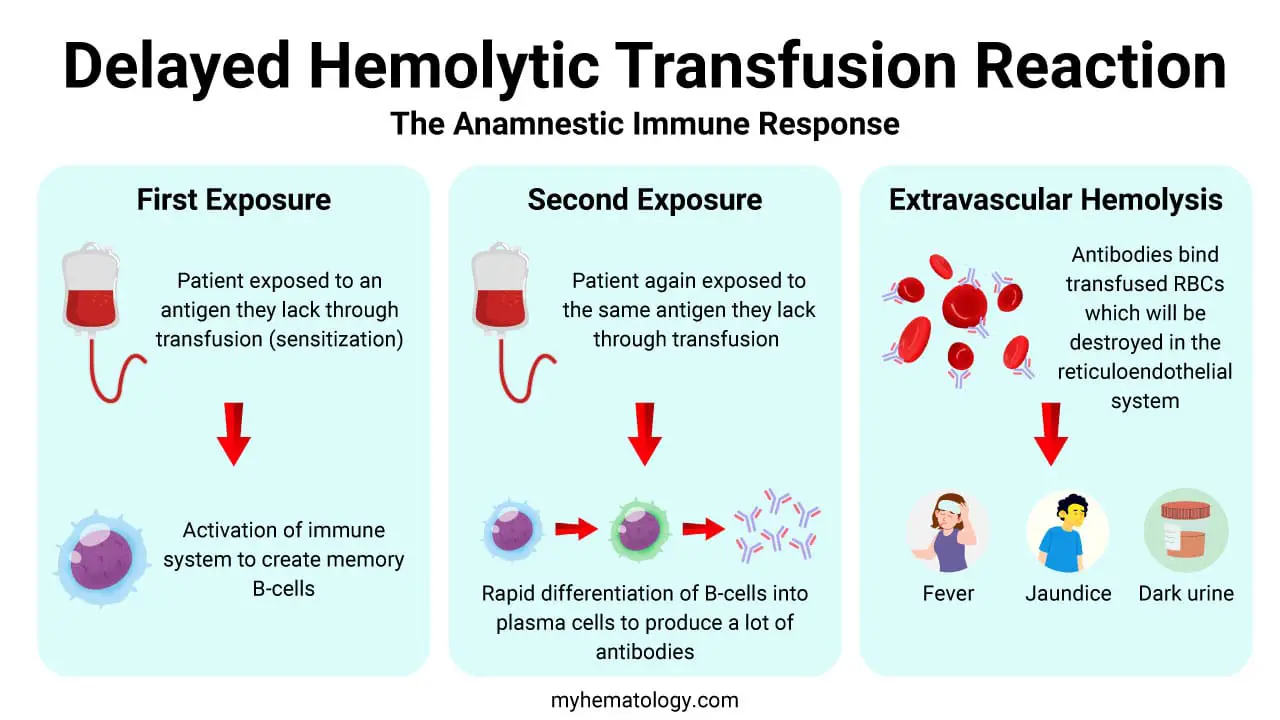
Initial Sensitization (The First Exposure)
This is the event that primes the recipient’s immune system. A patient may be exposed to an antigen they lack through a previous transfusion, a pregnancy (where fetal red blood cells enter the maternal circulation), or an organ transplant. This initial exposure is often uneventful clinically.
It leads to the activation of the immune system, resulting in the development of memory B-lymphocytes specific to that foreign antigen. These memory cells can persist for years, even decades, but the circulating antibody levels (titers) may be too low to be detected by routine pre-transfusion antibody screening tests.
The Anamnestic Response (The Second Exposure)
This is the key event that causes the clinical reaction. When the patient receives a second transfusion containing the same red blood cell antigen, their immune system rapidly recognizes it.
Antigen-presenting cells like dendritic cells (DCs), which are highly effective at capturing and processing antigens, take up the foreign red blood cells. They then present these processed antigens to the pre-existing memory T and B lymphocytes in the lymph nodes.
This leads to a rapid proliferation and differentiation of the memory B-lymphocytes into plasma cells. These plasma cells are highly efficient antibody factories, producing a large quantity of a specific type of antibody: immunoglobulin G (IgG). The production of these antibodies takes several days, which accounts for the delayed onset of the clinical symptoms.
Extravascular Hemolysis
Once a sufficient concentration of IgG antibodies is produced, they begin to bind to the surface of the transfused red blood cells that carry the target antigen. The resulting antibody-antigen complex is then recognized by macrophages in the reticuloendothelial system, primarily in the spleen and liver.
Macrophages have receptors that bind to the Fc portion of the IgG antibodies. Upon binding, the macrophages phagocytose (engulf and destroy) the antibody-coated red blood cells. This process, which occurs outside the blood vessels, is known as extravascular hemolysis. The destruction of the red blood cells releases hemoglobin, which is then metabolized into bilirubin, leading to the clinical finding of jaundice. This slow, but steady, destruction of transfused red cells is also why a delayed hemolytic transfusion reaction leads to a less dramatic and delayed drop in hemoglobin compared to acute reactions.
The most common antibodies implicated in delayed hemolytic transfusion reaction are those against antigens in the Kidd (Jk), Rh, Duffy (Fy), and Kell (K) blood group systems, with anti-Jka and anti-Jkb. Antibodies from the Kidd and Duffy systems are particularly notorious for causing delayed hemolytic transfusion reaction because they often become undetectable over time (their titers fall below the level of detection in routine antibody screening), only to be rapidly reactivated upon re-exposure to the corresponding antigen
Clinical Presentation and Symptoms
The clinical presentation of delayed hemolytic transfusion reaction can be subtle and is often mistaken for the underlying medical condition requiring the transfusion.
- Timing: Symptoms typically appear 3 to 10 days post-transfusion, though they can occur up to 28 days later.
- Signs of Hemolysis:
- Unexplained decrease in hemoglobin/hematocrit: The most common and often the only sign. The expected post-transfusion rise in hemoglobin is not sustained, or there is a sudden, unexpected drop.
- Fever: Often low-grade and without an infectious source.
- Jaundice or scleral icterus: Resulting from unconjugated hyperbilirubinemia.
- Dark urine: Due to hemoglobinuria.
- Other Symptoms:
- Malaise, fatigue, and chills.
Laboratory Investigation and Diagnosis of Delayed Hemolytic Transfusion Reaction
The clinical diagnosis of a delayed hemolytic transfusion reaction can be challenging due to its delayed and often subtle presentation. Therefore, a definitive diagnosis relies heavily on a series of key laboratory investigations. These tests serve two main purposes: to confirm that hemolysis is occurring and to identify the immune cause of the reaction.
Initial Laboratory Investigations (Evidence of Hemolysis)
These are the first tests typically ordered when delayed hemolytic transfusion reaction is suspected. They provide biochemical evidence of red blood cell destruction.
- Complete Blood Count (CBC) with Reticulocyte Count: A decline in the patient’s hemoglobin and hematocrit is a key finding, often accompanied by a low-grade fever. The reticulocyte count will typically be elevated as the bone marrow attempts to compensate for the ongoing hemolysis by producing new red blood cells.
- Indirect Bilirubin: The breakdown of heme from destroyed red blood cells leads to the production of unconjugated (indirect) bilirubin. A significant increase in indirect bilirubin is a strong indicator of hemolysis.
- Lactate Dehydrogenase (LDH): LDH is an enzyme found inside red blood cells. When hemolysis occurs, LDH is released into the bloodstream, leading to elevated serum levels.
- Haptoglobin: Haptoglobin is a protein that binds to free hemoglobin in the blood. In hemolytic states, haptoglobin levels are typically low or absent as it is consumed in the process of clearing hemoglobin.
Specific Serological Testing (The Gold Standard)
These tests are performed to confirm the immune nature of the reaction. Comparing pre-transfusion and post-transfusion samples is absolutely critical for diagnosis.
- Direct Antiglobulin Test (DAT) / Direct Coombs Test: This is the most important diagnostic test for delayed hemolytic transfusion reaction. The test detects antibodies that have already attached to the surface of the patient’s red blood cells. In a case of delayed hemolytic transfusion reaction, the DAT will be positive because the transfused red cells are coated with the recipient’s newly produced alloantibodies. A positive DAT in a patient with a history of recent transfusion is a strong indicator of an immune reaction.
- Antibody Screen and Panel: This test identifies specific antibodies in the patient’s plasma. The key finding in delayed hemolytic transfusion reaction is the presence of a newly appearing alloantibody or a significant increase in the titer of a pre-existing, weak antibody. The antibody panel is then used to identify the specific antigen(s) that the antibody is directed against (e.g., Kidd, Duffy, Kell). The demonstration of this new or strengthened antibody, which corresponds to an antigen present on the transfused unit, confirms the diagnosis.
Management of Delayed Hemolytic Transfusion Reaction
The management of delayed hemolytic transfusion reaction is primarily supportive.
Cessation of Transfusion
If the reaction is suspected, all transfusions must be stopped immediately if it is still ongoing.
Supportive Care
- Monitor Vitals: Closely monitor for signs of renal impairment or shock, though these are rare.
- Fluid Management: Maintain adequate hydration to prevent renal tubular damage from hemoglobinuria.
- Monitor Hemoglobin: Continue to monitor hemoglobin levels. Further transfusion may be necessary if the patient becomes symptomatic from anemia.
Future Transfusion Strategy
- Identify the responsible antibody: The patient’s blood bank record must be updated with the newly identified alloantibody.
- Antigen-negative blood: All future transfusions must consist of red blood cells that are negative for the corresponding antigen. This is crucial to prevent a more severe or recurrent reaction.
- Collaboration: Close collaboration with the hospital’s transfusion medicine service or blood bank is essential to ensure the safety of future transfusions.
Prognosis and Prevention of Delayed Hemolytic Transfusion Reaction
Prognosis and Clinical Outcome
The majority of delayed hemolytic transfusion reactions are considered benign and self-limiting. Patients typically experience a gradual decline in their hemoglobin, which may be detected only on a follow-up CBC. The symptoms are often mild, and the patient may not require any specific treatment beyond supportive care.
However, in a small minority of cases, delayed hemolytic transfusion reactions can lead to serious complications, including:
- Severe Anemia: In a patient with an already compromised hematological state (e.g., sickle cell disease), even a moderate drop in hemoglobin can be clinically significant and may necessitate further transfusions.
- Hyperbilirubinemia and Jaundice: While typically a benign sign, very high levels of bilirubin can be a concern, particularly in patients with liver dysfunction.
- Renal Failure: In rare, severe cases, the large amount of free hemoglobin released from hemolysis can overwhelm the body’s clearance mechanisms and lead to acute tubular necrosis and renal failure.
The key takeaway is that while most cases are mild, the potential for serious outcomes means that all suspected delayed hemolytic transfusion reactions must be investigated and managed appropriately.
Prevention
Prevention is the most critical aspect of managing delayed hemolytic transfusion reaction. Since the vast majority of cases are due to a secondary immune response, the goal is to identify and manage patients who have been previously sensitized to red blood cell antigens.
- Thorough Patient History: This is the first and most important step. Always ask patients about a history of:
- Previous transfusions
- Prior pregnancies
- Organ or stem cell transplants
- Pre-Transfusion Antibody Screening: Every patient should have a pre-transfusion antibody screen. While a negative screen does not guarantee a patient is free of alloantibodies (as titers may be too low to detect), it remains the cornerstone of transfusion safety.
- Phenotypically Matched Blood: For patients who are at a high risk of developing new alloantibodies, such as those with sickle cell disease or thalassemia who receive frequent transfusions, using phenotypically matched red blood cells is a vital preventive strategy. This means selecting donor units that lack the specific antigens to which the recipient is most likely to become sensitized (e.g., C, E, and Kell antigens).
- Alerting and Documentation: When a new alloantibody is identified, it is absolutely essential to flag the patient’s record in the hospital’s electronic health system. This ensures that all future transfusions will be with antigen-negative blood, preventing a repeat reaction. This documentation is a critical safety practice that prevents future harm.
- Universal Leukoreduction: While primarily intended to prevent febrile non-hemolytic transfusion reactions, leukoreduction may also reduce the risk of alloimmunization by removing donor white blood cells that present antigens.
Conclusion
Delayed hemolytic transfusion reaction is a significant cause of transfusion-related morbidity, often presenting as a subtle decline in hemoglobin levels days after a transfusion. An understanding of its anamnestic pathophysiology is key to early diagnosis, which relies on a positive DAT and the detection of a new alloantibody. While management is primarily supportive, the most crucial step is to update the patient’s record to ensure all future transfusions are with antigen-negative red cells. By implementing stringent pre-transfusion protocols and maintaining a high index of suspicion, medical professionals can significantly improve transfusion safety and patient outcomes.
Frequently Asked Questions (FAQs)
What is the difference between an acute and a delayed hemolytic transfusion reaction?
An acute hemolytic transfusion reaction (AHTR) occurs within 24 hours of a transfusion and is typically caused by pre-existing, clinically significant antibodies (usually ABO-mismatched). It is a rapid, often life-threatening reaction.
A delayed hemolytic transfusion reaction (DHTR) occurs 3 – 10 days post-transfusion and is due to an anamnestic (recall) immune response to an antigen the patient was previously exposed to, with the antibody level too low to be detected at the time of pre-transfusion testing.
Can a patient experience both a DHTR and a delayed serologic transfusion reaction (DSTR)?
A DSTR is a subclinical variant of DHTR. In DSTR, the alloantibody appears after the transfusion, but there are no clinical signs or symptoms of hemolysis (e.g., no significant drop in hemoglobin). A DHTR is a DSTR with clinical and laboratory evidence of hemolysis. Therefore, a DHTR is a subset of DSTR.
How do you differentiate a DHTR from a fever caused by infection?
While both can cause fever, a DHTR-related fever is typically not associated with a specific source of infection (e.g., cough, urinary symptoms). Laboratory workup for delayed hemolytic transfusion reaction will show signs of hemolysis (e.g., decreased hemoglobin, increased LDH and bilirubin), which would not be present in an isolated infectious fever. A positive DAT is a key distinguishing feature.
Glossary of Medical Terms
- Alloantibody: An antibody produced by an individual against an antigen from another individual of the same species. In transfusion, these are antibodies against foreign blood group antigens.
- Anamnestic Response: A rapid and heightened immune response that occurs after a second or subsequent encounter with the same antigen. Also known as a “recall response.”
- Direct Antiglobulin Test (DAT): A test that detects antibodies (e.g., IgG) or complement components bound to the surface of red blood cells in vivo. A positive result indicates that the patient’s red blood cells are coated with antibodies. Also known as the Coombs test.
- Extravascular Hemolysis: The destruction of red blood cells that occurs outside of the bloodstream, primarily within the reticuloendothelial system (spleen, liver, and bone marrow).
- Hemoglobinuria: The presence of free hemoglobin in the urine, resulting from the destruction of red blood cells.
- Reticuloendothelial System (RES): A part of the immune system that consists of phagocytic cells, primarily macrophages, that are responsible for the removal of old cells, foreign particles, and immune complexes from the circulation.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- American Association of Blood Banks (AABB). Technical Manual, 21st Edition, 2023.
- Dean L. Blood Groups and Red Cell Antigens [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2005.
- Rout P, Harewood J, Ramsey A, et al. Hemolytic Transfusion Reaction. [Updated 2023 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448158/
- Thonier V. (2019). Immuno-hematological findings in Delayed Hemolytic Transfusion Reaction (DHTR). Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine, 26(2), 102–108. https://doi.org/10.1016/j.tracli.2019.02.006
- Hendrickson, J. E., & Fasano, R. M. (2021). Management of hemolytic transfusion reactions. Hematology. American Society of Hematology. Education Program, 2021(1), 704–709. https://doi.org/10.1182/hematology.2021000308
- Pirenne F. (2019). Prevention of delayed hemolytic transfusion reaction. Transfusion clinique et biologique : journal de la Societe francaise de transfusion sanguine, 26(2), 99–101. https://doi.org/10.1016/j.tracli.2019.02.007
- Delaney, M., Wendel, S., Bercovitz, R. S., Cid, J., Cohn, C., Dunbar, N. M., Apelseth, T. O., Popovsky, M., Stanworth, S. J., Tinmouth, A., Van De Watering, L., Waters, J. H., Yazer, M., Ziman, A., & Biomedical Excellence for Safer Transfusion (BEST) Collaborative (2016). Transfusion reactions: prevention, diagnosis, and treatment. Lancet (London, England), 388(10061), 2825–2836. https://doi.org/10.1016/S0140-6736(15)01313-6
- Alwaheed, A. J., Alqatari, S. G., AlSulaiman, A. S., & AlSulaiman, R. S. (2022). Delayed Hemolytic Transfusion Reaction in Sickle Cell Disease: A Case Series. The American journal of case reports, 23, e934681. https://doi.org/10.12659/AJCR.934681