TL;DR
Hemolytic uremic syndrome is defined by the triad of microangiopathic hemolytic anemia, acute kidney injury, and thrombocytopenia. It’s a serious, potentially life-threatening condition.
- Classification ▾: Primarily classified as STEC-HUS (typical, often post-diarrheal), atypical HUS (aHUS, complement dysregulation), and secondary HUS (associated with other conditions).
- Causes & Pathophysiology:
- STEC-HUS: Shiga toxins from E. coli damage endothelial cells, leading to microthrombi.
- aHUS: Uncontrolled activation of the alternative complement pathway damages endothelium.
- Secondary HUS: Arises from various underlying conditions (infections, drugs, etc.).
- Symptoms ▾: Include pallor, fatigue, decreased urine output, edema, bruising, and potentially neurological or gastrointestinal issues. STEC-HUS often has a preceding diarrheal prodrome.
- Laboratory Investigations ▾: Key findings include anemia with schistocytes, thrombocytopenia, elevated creatinine/BUN, and potentially positive stool/toxin tests (STEC-HUS) or abnormal complement studies (aHUS).
- Treatment & Management ▾: Primarily supportive care (fluids, electrolytes, transfusions, antihypertensives). aHUS may require plasma exchange or complement inhibitors (eculizumab, ravulizumab). Kidney replacement therapy may be needed.
- Complications ▾: Acute complications include severe AKI, neurological issues, hypertension, and bowel problems. Long-term complications can include CKD, hypertension, and neurocognitive deficits.
- Prognosis ▾: Varies by HUS type. STEC-HUS generally has a good prognosis with support. aHUS prognosis has improved with complement inhibitors. Secondary HUS prognosis depends on the underlying condition.
- Prevention ▾: For STEC-HUS, focus is on food safety and hygiene. aHUS prevention is challenging due to genetic factors. Secondary HUS prevention involves managing underlying conditions.
*Click ▾ for more information
Introduction
Hemolytic uremic syndrome is a serious condition characterized by a triad of microangiopathic hemolytic anemia (damage and destruction of red blood cells), acute kidney injury (sudden decrease in kidney function), and thrombocytopenia (low blood platelet count).
Hemolytic uremic syndrome (HUS) can be life-threatening due to the severe damage it causes to vital organs, primarily the kidneys and brain. The acute kidney injury can rapidly lead to kidney failure, requiring dialysis and potentially kidney transplantation. The microangiopathic hemolytic anemia can cause severe anemia, leading to organ damage from lack of oxygen. Furthermore, the associated low platelet count can result in dangerous bleeding. Neurological complications, such as seizures, stroke, and coma, can also arise from the systemic effects of the disease, significantly increasing the risk of mortality and long-term disability.
Epidemiology
Hemolytic uremic syndrome (HUS) is most common in young children, particularly those under 5 years of age. The incidence of Shiga toxin-producing E. coli (STEC)-HUS peaks in children younger than 5 years, with estimates around 2.1 to 6.1 cases per 100,000 per year in this age group. Some studies report even higher rates in specific age ranges within this group, such as 6 months to 4 years.
In contrast, HUS is less common in adults, with the lowest rates observed in those aged 50-59 years.
Atypical HUS (aHUS) can occur at any age, and its incidence in children is estimated to be around 0.2 per 100,000 total population per year in the United States.
Variations Based on Geographic Location
The incidence of hemolytic uremic syndrome shows significant geographic variation, likely influenced by factors such as hygiene practices, agricultural practices (especially cattle raising), and the prevalence of Shiga toxin-producing E. coli (STEC) infections.
North America and Europe report annual incidences of STEC-HUS in young children (under 5 years) of approximately 2 to 3 per 100,000. Argentina has reported particularly high rates of STEC-HUS, reaching 60 to 120 per million in children under 5 years.
Studies have shown variations even within countries, with some suggesting that STEC-HUS might be more common in rural areas compared to urban areas, possibly due to differences in hygiene and exposure to STEC reservoirs.
Colder regions have been reported to have a higher incidence compared to warmer climates, and the incidence tends to parallel the seasonal fluctuation of E. coli O157:H7 infection, peaking in warmer months (June to September in the Northern Hemisphere).
Classification of Hemolytic Uremic Syndrome
Hemolytic Uremic Syndrome (HUS) is primarily classified based on its underlying cause and clinical presentation. The two main categories are Shiga toxin-producing Escherichia coli (STEC)-HUS (typical HUS, D+HUS) and atypical HUS (aHUS, D-HUS). Additionally, there’s a third category, secondary HUS, which arises in association with other underlying conditions.
Shiga Toxin-Producing Escherichia coli HUS (STEC-HUS) – Typical HUS (D+HUS)
This is the most common form of hemolytic uremic syndrome, accounting for the majority of cases, especially in children. It is strongly associated with infection by Shiga toxin-producing bacteria, most notably Escherichia coli serotype O157:H7.
- Causative Agent: Primarily caused by Shiga toxins (Stx1 and Stx2) produced by certain strains of E. coli. Other STEC serotypes (non-O157) are also increasingly recognized as causes.
- Preceding Illness: Typically preceded by a prodromal diarrheal illness, often bloody, accompanied by abdominal cramps and vomiting. This gastrointestinal phase usually occurs a few days to a week before the onset of hemolytic uremic syndrome (HUS) symptoms.
- Pathophysiology: The Shiga toxins are absorbed from the gut and enter the bloodstream. They then bind to specific receptors (globotriaosylceramide, Gb3) on endothelial cells, particularly in the glomeruli of the kidneys. This binding leads to endothelial cell damage, activation of the coagulation cascade, and the formation of microthrombi in the small blood vessels. The mechanical shearing of red blood cells as they pass through these narrowed and damaged vessels results in microangiopathic hemolytic anemia. Platelets are consumed in the microthrombi, leading to thrombocytopenia.
- Age Group: Predominantly affects young children.
- Prognosis: Generally has a better prognosis compared to aHUS with supportive care. Most patients recover kidney function, although some may have long-term sequelae.
Atypical Hemolytic Uremic Syndrome (aHUS) – (D-HUS)
This form of hemolytic uremic syndrome is not associated with a preceding diarrheal illness caused by STEC. It is characterized by dysregulation of the alternative pathway of the complement system, a crucial part of the innate immune system.
- Causative Mechanisms:
- Genetic Mutations: In approximately 50-70% of cases, aHUS is linked to genetic mutations in genes encoding complement regulatory proteins (e.g., complement factor H (CFH), complement factor I (CFI), membrane cofactor protein (MCP or CD46), complement factor B (CFB), complement component 3 (C3), thrombomodulin (THBD)). These mutations lead to uncontrolled activation of the alternative complement pathway on endothelial surfaces.
- Acquired Factors: In some cases, aHUS can be caused by acquired factors, such as autoantibodies that interfere with complement regulation (e.g., anti-factor H antibodies).
- Preceding Illness: Typically no preceding diarrheal illness. However, it can sometimes be triggered by other infections (viral or bacterial), vaccinations, or pregnancy.
- Pathophysiology: Uncontrolled activation of the alternative complement pathway on the surface of endothelial cells leads to their damage and activation. This results in inflammation, the formation of microthrombi, microangiopathic hemolytic anemia, and thrombocytopenia, similar to STEC-HUS but driven by a different initial mechanism. The kidneys are often the primary target, but other organs like the brain, heart, and gastrointestinal tract can also be affected.
- Age Group: Can occur at any age, from infancy to adulthood.
- Prognosis: Historically had a poorer prognosis with higher rates of kidney failure and mortality compared to STEC-HUS. However, the advent of complement inhibitors like eculizumab has significantly improved outcomes. Early diagnosis and treatment are crucial.
Secondary Hemolytic Uremic Syndrome
This category encompasses cases of hemolytic uremic syndrome that develop in association with or secondary to other underlying medical conditions or exposures.
- Associated Conditions/Factors: A wide range of conditions can be associated with secondary hemolytic uremic syndrome (HUS), including:
- Infections: Certain non-STEC bacterial infections (e.g., Streptococcus pneumoniae producing neuraminidase), viral infections (e.g., HIV, influenza).
- Medications: Some drugs, such as calcineurin inhibitors (e.g., cyclosporine, tacrolimus), certain chemotherapeutic agents (e.g., mitomycin C, gemcitabine), and antiplatelet drugs.
- Pregnancy: Particularly associated with severe preeclampsia and HELLP syndrome (Hemolysis, Elevated Liver enzymes, and Low Platelet count).
- Autoimmune Diseases: Systemic lupus erythematosus (SLE), antiphospholipid syndrome.
- Malignancy: Certain cancers, particularly metastatic disease.
- Stem Cell Transplantation: Often related to graft-versus-host disease or calcineurin inhibitor use.
- Metabolic Disorders: Cobalamin deficiency.
- Pathophysiology: The mechanisms leading to hemolytic uremic syndrome in these cases vary depending on the underlying condition. For example, Streptococcus pneumoniae-associated hemolytic uremic syndrome involves the action of neuraminidase on red blood cell antigens, leading to platelet activation and aggregation. Drug-induced hemolytic uremic syndrome may involve direct endothelial toxicity.
- Treatment: Management focuses on addressing the underlying cause while providing supportive care for the hemolytic uremic syndrome. Specific therapies may be needed depending on the primary condition.
- Prognosis: The prognosis of secondary hemolytic uremic syndrome is highly variable and depends largely on the severity and treatability of the underlying condition.
While the clinical manifestations of hemolytic uremic syndrome share common features (hemolytic anemia, thrombocytopenia, and acute kidney injury), the underlying causes and the precise molecular pathways leading to endothelial damage and thrombotic microangiopathy differ significantly between STEC-HUS, aHUS, and secondary HUS. Understanding these distinctions is crucial for accurate diagnosis and the development of targeted therapies.
Signs and Symptoms of Hemolytic Uremic Syndrome
The signs and symptoms of hemolytic uremic syndrome can vary in severity and presentation, but they generally reflect the underlying triad of hemolytic anemia, thrombocytopenia, and acute kidney injury, as well as potential involvement of other organ systems.
Prodromal Phase (Primarily in STEC-HUS)
- Diarrhea: Often the initial symptom, typically starting as watery and progressing to bloody diarrhea. This is a key distinguishing feature of STEC-HUS.
- Abdominal Cramps: Significant abdominal pain and cramping are common during the diarrheal phase.
- Vomiting: Nausea and vomiting may accompany the diarrhea.
- Fever: A mild fever might be present during the prodromal phase, but it’s usually not high or prolonged.
- Duration: This prodromal phase typically lasts for a few days (usually 5-10 days) before the onset of the classic HUS triad. It’s important to note that aHUS typically does not have this preceding diarrheal illness.
Classic Triad Symptoms
As the prodromal symptoms (if present) subside or in the absence of them (in aHUS), the hallmark signs of hemolytic uremic syndrome emerge.
- Hemolytic Anemia
- Pallor
- Fatigue and Weakness
- Irritability and Dizziness
- Jaundice (mild)
- Acute Kidney Injury (AKI)
- Decreased Urine Output (Oliguria): Producing less urine than usual is a significant indicator of impaired kidney function. In severe cases, there might be no urine output (anuria).
- Edema: Swelling, particularly in the legs, ankles, face, and around the eyes, due to fluid retention by the failing kidneys.
- Hypertension: High blood pressure can develop as the kidneys lose their ability to regulate fluid and electrolytes.
- Hematuria: Blood in the urine, which may be visible (gross hematuria) or only detectable under a microscope (microscopic hematuria).
- Changes in Urine Appearance: Urine may appear darker or tea-colored.
- Thrombocytopenia
- Bruising (Ecchymoses): Easy bruising or large, unexplained bruises on the skin.
- Petechiae: Small, pinpoint red or purple spots on the skin, often resembling a rash, due to bleeding under the skin. These are commonly seen on the chest, arms, and legs.
- Bleeding: Increased tendency to bleed, such as nosebleeds (epistaxis), gum bleeding, or prolonged bleeding from minor cuts.
Systemic Involvement (Beyond the Classic Triad)
Hemolytic uremic syndrome can affect other organ systems, leading to a variety of additional symptoms.
- Neurological Manifestations: These can range from mild to severe and are more common in severe cases.
- Irritability and Lethargy
- Confusion and Disorientation
- Seizures
- Stroke (rare but serious)
- Coma (in severe cases)
- Gastrointestinal Manifestations (Beyond the Prodrome in STEC-HUS)
- Persistent Abdominal Pain and Tenderness.
- Colitis: Inflammation of the colon can cause ongoing abdominal pain and sometimes further bloody stools.
- Intussusception (rare complication, especially in children): Telescoping of one part of the intestine into another, causing severe abdominal pain, vomiting, and potentially bloody, mucus-containing stools (“currant jelly” stools).
- Cardiovascular Manifestations
- Hypertension.
- Rarely, heart failure due to fluid overload and severe anemia.
- Pancreatic Involvement: Pancreatitis (inflammation of the pancreas) can occur in some cases, leading to severe abdominal pain and vomiting.
Young children may present with more non-specific symptoms initially, such as fussiness, poor feeding, and lethargy. Hemolytic uremic syndrome in adults, however, may have a different presentation and may be less likely to have the classic diarrheal prodrome, especially in aHUS and secondary hemolytic uremic syndrome.
Recognizing these signs and symptoms, particularly the classic triad in the appropriate clinical context (especially after a diarrheal illness in children), is crucial for early diagnosis and prompt management of hemolytic uremic syndrome. The presence of neurological symptoms often indicates more severe disease.
Laboratory Investigations of Hemolytic Uremic Syndrome
A comprehensive panel of tests is typically performed to assess the hematological, renal, and coagulation systems, as well as to identify the underlying cause, especially distinguishing between STEC-HUS and aHUS.
Hematological Studies
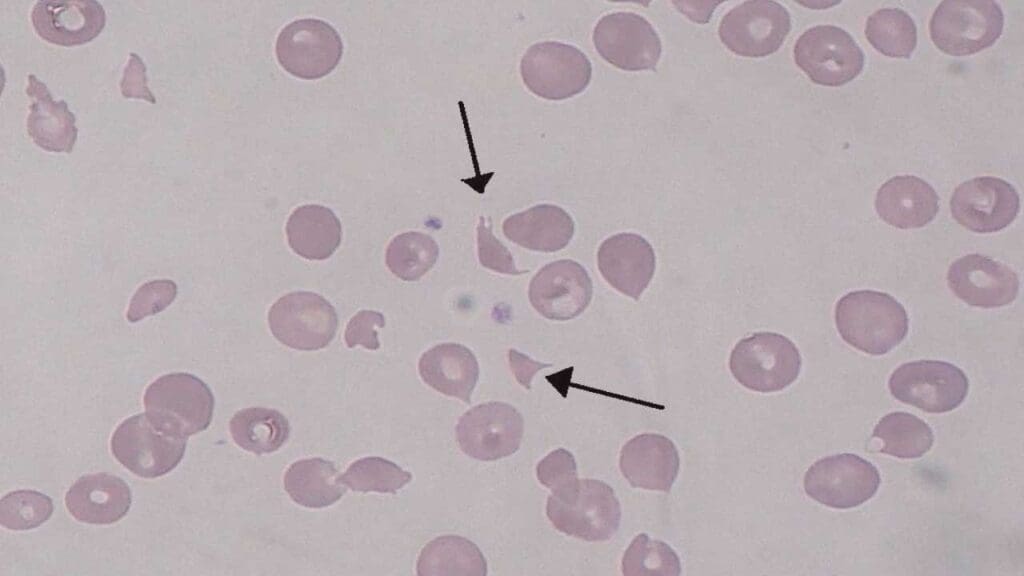
- Complete Blood Count (CBC) with Peripheral Blood Smear
- Hemoglobin and Hematocrit: Low, indicating anemia. The severity of anemia can vary.
- Platelet Count: Usually below 150,000/µL and often significantly lower (thrombocytopenia). This reflects platelet consumption in microthrombi.
- White Blood Cell (WBC) Count: May be normal or slightly elevated. A significant increase might suggest a concurrent infection or inflammatory process.
- Peripheral Blood Smear: This is a critical test. It will reveal schistocytes (fragmented red blood cells, often helmet-shaped), which are a hallmark of microangiopathic hemolytic anemia, resulting from the mechanical damage to red blood cells as they pass through the narrowed and damaged small blood vessels. Polychromasia (increased number of immature red blood cells or reticulocytes) may also be seen, indicating the bone marrow’s attempt to compensate for the anemia.
- Reticulocyte Count: Elevated, indicating increased red blood cell production by the bone marrow in response to the hemolysis.
- Direct Antiglobulin Test (DAT or Coombs Test): In typical STEC-HUS and atypical HUS, the DAT is usually negative, helping to differentiate HUS from autoimmune hemolytic anemia. However, in some rare cases of secondary HUS associated with autoimmune conditions, the DAT might be positive.
- Lactate Dehydrogenase (LDH): LDH levels will be elevated, reflecting the ongoing hemolysis.
- Haptoglobin: In hemolytic uremic syndrome, haptoglobin levels will be low or undetectable due to the increased consumption of haptoglobin in binding the released hemoglobin.
Renal Function Tests
- Serum Creatinine and Blood Urea Nitrogen (BUN): In acute kidney injury, their levels will be elevated, indicating impaired glomerular filtration rate (GFR). The degree of elevation reflects the severity of kidney dysfunction.
- Estimated Glomerular Filtration Rate (eGFR): It provides a more direct assessment of kidney function and will be reduced in hemolytic uremic syndrome (HUS).
- Serum Electrolytes: Levels of electrolytes such as sodium, potassium, calcium, and phosphate may be abnormal due to impaired kidney function. Hyperkalemia (high potassium) is a particular concern as it can lead to cardiac arrhythmias.
- Urinalysis: Examination of the urine can reveal:
- Proteinuria: Presence of protein in the urine, indicating glomerular damage.
- Hematuria: Presence of red blood cells in the urine (microscopic or gross).
- Urinary Casts: Abnormal microscopic structures formed in the kidney tubules. Different types of casts can provide clues about the nature of kidney injury.
Stool Studies (Crucial for Suspected STEC-HUS)
- Stool Culture for E. coli O157:H7: If a diarrheal prodrome is present or STEC-HUS is suspected, stool culture should be performed to identify E. coli O157:H7. However, the organism may no longer be present by the time hemolytic uremic syndrome (HUS) develops, as the toxin has already been released and absorbed.
- Shiga Toxin Testing (EIA or PCR) of Stool Samples: Even if the culture is negative for E. coli O157:H7, testing for Shiga toxins (Stx1 and Stx2) in the stool can be positive and is a more sensitive indicator of STEC infection as the cause of hemolytic uremic syndrome (HUS). PCR-based assays are increasingly used for their sensitivity and ability to detect non-O157 STEC strains as well.
Complement Studies (Essential for Suspected Atypical Hemolytic Uremic Syndrome)
If there is no evidence of STEC infection (negative stool culture and Shiga toxin assay) or if the clinical presentation is suggestive of aHUS (e.g., no preceding diarrhea, relapsing course, onset at any age), a comprehensive evaluation of the complement system is necessary. This may include:
- Measurement of Complement Component Levels: Levels of complement components like C3 and C4 are often measured. In aHUS due to alternative pathway dysregulation, C3 levels may be low due to increased consumption. C4 levels are usually normal as it is primarily involved in the classical and lectin pathways.
- Functional Assays of the Alternative Complement Pathway (e.g., AH50): In aHUS, it may show increased or unregulated activity.
- Genetic Testing for Mutations in Complement Regulatory Genes: Genetic testing for mutations in genes encoding complement factors and regulatory proteins (e.g., CFH, CFI, MCP/CD46, CFB, C3, THBD, DGKE) is crucial for confirming the diagnosis of aHUS and identifying the specific genetic defect. This can have implications for prognosis and family screening.
- Testing for Autoantibodies Against Complement Regulatory Proteins: Assays to detect autoantibodies, particularly anti-factor H antibodies, are important for diagnosing acquired forms of aHUS. Antibodies against other complement regulators like factor I and factor B can also occur, although less frequently.
Other Investigations (Depending on Clinical Presentation and Suspected Secondary Causes)
- Antistreptolysin O (ASO) titer and anti-DNase B antibodies: May be checked if post-streptococcal glomerulonephritis is in the differential diagnosis, although Streptococcus pneumoniae-associated hemolytic uremic syndrome has different mechanisms.
- Serological tests for other infections: If a specific infection is suspected as a trigger for secondary hemolytic uremic syndrome (e.g., HIV).
- Drug levels: If drug-induced hemolytic uremic syndrome is suspected (e.g., calcineurin inhibitor levels).
- Autoimmune workup: Including ANA, anti-dsDNA, antiphospholipid antibodies if an underlying autoimmune disease is suspected.
| Laboratory Parameter | STEC-HUS (Typical) | aHUS (Atypical) | Secondary HUS |
| Hematology | |||
| Hemoglobin | Low (anemia) | Low (anemia) | Low (anemia) |
| Platelet Count | Low (thrombocytopenia) | Low (thrombocytopenia) | Low (thrombocytopenia) |
| Peripheral Blood Smear | Schistocytes present | Schistocytes present | Schistocytes present |
| Reticulocyte Count | Elevated | Elevated | Elevated |
| DAT (Coombs Test) | Usually Negative | Usually Negative | May be positive depending on underlying cause |
| LDH | Elevated | Elevated | Elevated |
| Haptoglobin | Low/Undetectable | Low/Undetectable | Low/Undetectable |
| Renal Function | |||
| Serum Creatinine | Elevated | Elevated | Elevated |
| BUN | Elevated | Elevated | Elevated |
| Urinalysis | Proteinuria, hematuria, casts | Proteinuria, hematuria, casts | Proteinuria, hematuria, casts |
| Etiology-Specific Tests | |||
| Stool Culture for E. coli O157:H7 | May be Positive (early in illness) | Usually Negative | Negative |
| Shiga Toxin Assay (Stool) | May be Positive | Usually Negative | Negative |
| Complement Studies | Usually Normal | Abnormal in many cases: Low C3, abnormal AH50, genetic mutations, autoantibodies | Usually Normal (unless underlying cause affects complement) |
| Other | |||
| ASO/Anti-DNase B | Usually Normal | Usually Normal | May be elevated in post-strep HUS |
| Serology for other infections | Negative (unless secondary cause) | Negative (unless trigger) | May be positive depending on underlying infection |
| Drug levels | Normal | Normal | May be elevated if drug-induced |
| Autoimmune Markers | Usually Negative | Usually Negative | May be positive if associated with autoimmune disease |
Differential Diagnosis of Hemolytic Uremic Syndrome
Several other conditions can present with overlapping features of hemolytic anemia, thrombocytopenia, and kidney injury.
| Feature | HUS | TTP | DIC | HELLP Syndrome |
| Key Triad | Microangiopathic hemolytic anemia, thrombocytopenia, acute kidney injury (AKI) | Microangiopathic hemolytic anemia, thrombocytopenia (AKI less prominent) | Microangiopathic hemolytic anemia, thrombocytopenia (AKI variable) | Hemolysis, thrombocytopenia (elevated liver enzymes, AKI variable) |
| ADAMTS13 Activity | Usually Normal | Severely Deficient (<10%) | Usually Normal | Normal |
| Neurological Signs | Less common initially, can be severe later | Common and often prominent (altered mental status, seizures, etc.) | Variable, related to underlying cause | Rare |
| Fever | Mild or absent (except sometimes in prodrome of STEC-HUS) | Often present as part of the classic pentad | Variable, related to underlying cause | May be present |
| Preceding Diarrhea | Common in STEC-HUS, absent in aHUS | Rare | Related to underlying cause (e.g., sepsis) | Absent |
| Coagulation Studies (PT/aPTT, Fibrinogen, D-dimer) | Usually Normal or minimally deranged | Usually Normal | Often Abnormal (prolonged PT/aPTT, low fibrinogen, high D-dimer) | Usually Normal |
| Underlying Condition | Often STEC infection or complement dysregulation | Primary enzyme deficiency | Always secondary to another condition | Pregnancy or postpartum |
| Liver Enzymes | Usually Normal or mildly elevated (in severe aHUS) | Usually Normal | May be abnormal depending on underlying cause | Elevated (key feature of HELLP) |
| Complement Studies | Usually Normal in STEC-HUS, Abnormal in many aHUS cases | Usually Normal | Usually Normal | Usually Normal |
Treatment and Management of Hemolytic Uremic Syndrome
The treatment and management of hemolytic uremic syndrome are primarily focused on providing supportive care to address the complications of hemolytic anemia, thrombocytopenia, and acute kidney injury. Specific therapies may be indicated depending on the type of HUS (STEC-HUS, aHUS, or secondary HUS).
General Supportive Care (Essential for All Types of HUS)
- Fluid and Electrolyte Management: Careful monitoring of fluid balance is crucial. Patients may be oliguric (producing little urine) and prone to fluid overload, which can lead to hypertension, edema, and even heart failure. Intravenous fluids are often necessary but must be administered judiciously, with close monitoring of urine output, weight, and signs of fluid overload. Electrolyte imbalances (e.g., hyperkalemia, hyponatremia, hypocalcemia, hyperphosphatemia) are common due to kidney dysfunction and need to be monitored and corrected promptly to prevent cardiac arrhythmias and other complications.
- Blood Transfusions: Packed red blood cell transfusions may be necessary to treat severe anemia and improve oxygen delivery. However, they should be given cautiously to avoid exacerbating fluid overload and hypertension.
- Platelet Transfusions: Generally avoided unless there is active, significant bleeding or a planned invasive procedure. In hemolytic uremic syndrome, platelet consumption is ongoing due to microthrombi formation, and transfused platelets are likely to be rapidly consumed, providing only transient benefit and potentially increasing the risk of thrombotic complications in some contexts.
- Antihypertensive Therapy: Hypertension is common, especially with fluid overload and kidney dysfunction. Antihypertensive medications are often required to control blood pressure and prevent further kidney damage and neurological complications.
- Nutritional Support: Patients with hemolytic uremic syndrome, especially those with gastrointestinal symptoms or kidney failure, may have poor oral intake. Nutritional support, either enteral (via nasogastric or gastrostomy tube) or parenteral (intravenous nutrition), may be necessary to maintain adequate caloric and protein intake.
- Seizure Management: If neurological complications such as seizures occur, they need to be treated with appropriate anticonvulsant medications.
- Monitoring for Complications: Close monitoring for other potential complications such as pancreatitis, bowel ischemia/perforation, and cardiac issues is essential.
Specific Therapies Based on the Type of Hemolytic Uremic Syndrome
STEC-HUS (Typical HUS)
- No Specific Anti-Toxin Therapy: Currently, there is no specific treatment to directly neutralize Shiga toxins once they are absorbed.
- Avoid Antibiotics: Antibiotics are generally avoided during the acute diarrheal phase of STEC-HUS. Studies suggest that antibiotic use may increase the release of Shiga toxins from the bacteria, potentially worsening the severity of HUS.
- Focus on Supportive Care: Management is primarily focused on meticulous supportive care to manage the complications of anemia, thrombocytopenia, and acute kidney injury until the illness resolves spontaneously, which typically occurs within a few weeks.
Atypical Hemolytic Uremic Syndrome (aHUS)
- Plasma Exchange (PEX) or Plasma Infusion (PI): Historically, PEX was often the first-line treatment for suspected aHUS. The goal is to remove any circulating autoantibodies or abnormal complement factors and replace deficient complement regulatory proteins with those present in normal plasma. PEX can be intensive, requiring daily or every-other-day treatments. Plasma infusion may be considered in some situations.
- Eculizumab: This is a recombinant humanized monoclonal antibody that binds to the complement protein C5, inhibiting its cleavage into C5a and C5b, and thus preventing the formation of the membrane attack complex (MAC). Eculizumab has significantly improved outcomes in aHUS and is often the first-line targeted therapy. It requires regular intravenous infusions. Vaccination against Neisseria meningitidis is crucial before starting eculizumab due to the increased risk of meningococcal infection with C5 inhibition.
- Ravulizumab: A longer-acting C5 inhibitor that allows for less frequent dosing compared to eculizumab. It has also shown efficacy in treating aHUS.
- Other Emerging Therapies: Research is ongoing, and other complement inhibitors targeting different points in the pathway are being developed and used in some cases.
- Genetic Testing Guidance: Identifying the specific genetic mutation in aHUS can sometimes influence treatment duration and the likelihood of recurrence.
Secondary HUS
- Treating the Underlying Cause: The primary focus of treatment is to identify and manage the underlying condition that triggered the hemolytic uremic syndrome (e.g., treating the specific infection, discontinuing the offending medication, managing the autoimmune disease or malignancy).
- Supportive Care: Supportive care for the hematological and renal complications is also essential.
- Plasma Exchange: May be beneficial in some cases of secondary hemolytic uremic syndrome, depending on the underlying cause.
Kidney Replacement Therapy (KRT)
- Indications: Dialysis (either hemodialysis or peritoneal dialysis) may be necessary in cases of severe acute kidney injury with:
- Severe oliguria or anuria (little to no urine production).
- Unmanageable fluid overload.
- Severe hyperkalemia.
- Metabolic acidosis unresponsive to medical management.
- Symptomatic uremia (e.g., pericarditis, encephalopathy).
- Duration: The need for dialysis can be temporary, and kidney function may recover, particularly in STEC-HUS. However, some patients, especially those with aHUS or severe secondary HUS, may develop chronic kidney disease and require long-term dialysis or kidney transplantation.
Long-Term Management and Follow-Up
- Monitoring for Chronic Kidney Disease (CKD): Even after recovery from the acute phase, patients need long-term follow-up to monitor for the development of CKD, hypertension, and proteinuria.
- Blood Pressure Control: Aggressive management of hypertension is crucial to preserve kidney function.
- Urine Studies: Regular monitoring of urine protein levels.
- Growth and Development in Children: For children who have had hemolytic uremic syndrome, long-term monitoring of growth and development is important.
- Recurrence Risk: Patients with aHUS, especially those with specific genetic mutations, may have a higher risk of recurrence, requiring ongoing monitoring and potentially prophylactic treatment.
Potential Complications of Hemolytic Uremic Syndrome
Hemolytic Uremic Syndrome is a severe condition that can lead to a range of acute and long-term complications affecting various organ systems. The likelihood and severity of these complications can vary depending on the type of hemolytic uremic syndrome (STEC-HUS, aHUS, or secondary HUS), the promptness and effectiveness of treatment, and the individual patient’s overall health.
Acute Complications (Occurring During the Active Phase of the Illness)
- Severe Acute Kidney Injury (AKI) Requiring Dialysis: This is a major acute complication. The damage to the small blood vessels in the kidneys can lead to a rapid and significant decline in kidney function, often necessitating temporary or, in some cases, permanent kidney replacement therapy (dialysis).
- Neurological Sequelae: These can range from mild to severe and can significantly impact the patient’s outcome.
- Seizures: Due to electrolyte imbalances, hypertension, or direct effects of toxins or complement activation on the brain.
- Stroke: Can occur due to blood clots forming in the brain’s blood vessels.
- Encephalopathy: A general term for brain dysfunction, which can manifest as altered mental status, confusion, lethargy, irritability, and coma.
- Focal Neurological Deficits: Weakness in a part of the body, speech difficulties, or visual disturbances can occur due to localized brain injury.
- Hypertension: High blood pressure can develop due to fluid overload from kidney dysfunction and activation of the renin-angiotensin-aldosterone system. Uncontrolled hypertension can further damage the kidneys and contribute to neurological complications.
- Pancreatitis: Inflammation of the pancreas can occur, possibly due to microthrombi in the pancreatic blood vessels or systemic inflammation. This can cause severe abdominal pain, nausea, and vomiting.
- Colonic Complications
- Colitis: Inflammation of the colon, which is part of the initial STEC infection, can become severe and lead to significant abdominal pain and bleeding.
- Bowel Ischemia and Perforation (Rare): In severe cases, reduced blood flow to the bowel due to microthrombi can lead to tissue damage, ischemia, and potentially perforation, a life-threatening complication.
- Intussusception (Rare): Especially in children with STEC-HUS, the inflammation can sometimes lead to intussusception, where one part of the intestine telescopes into another, causing obstruction and requiring medical or surgical intervention.
- Cardiac Complications (Rare): Myocarditis (inflammation of the heart muscle) or heart failure can occur due to severe anemia, fluid overload, or systemic inflammation, although these are less common acute complications.
- Severe Anemia Requiring Multiple Transfusions: While blood transfusions are part of the management, the ongoing hemolysis can be severe enough to require multiple transfusions, increasing the risk of transfusion-related complications (e.g., allergic reactions, fluid overload).
Long-Term Complications (Persisting After the Acute Illness)
- Chronic Kidney Disease (CKD): This is a significant long-term complication, with varying degrees of severity, ranging from mild kidney damage (e.g., persistent proteinuria or reduced GFR) to end-stage renal disease requiring long-term dialysis or kidney transplantation. The risk of CKD is higher in patients with more severe initial kidney injury and in those with aHUS.
- Hypertension: Persistent high blood pressure can occur even after kidney function recovers to some extent, likely due to residual kidney damage and dysregulation of blood pressure control mechanisms. Long-term hypertension can increase the risk of cardiovascular disease.
- Neurocognitive Deficits: Some patients, particularly those who experienced neurological complications during the acute phase, may have long-term neurocognitive deficits, such as learning disabilities, attention deficits, or memory problems.
- Increased Risk of Future Kidney Problems: Individuals who have had hemolytic uremic syndrome may have an increased susceptibility to developing kidney problems later in life, even if they recover good kidney function initially.
- Recurrence of aHUS: Patients with aHUS, especially those with underlying genetic mutations or persistent complement dysregulation, have a risk of disease recurrence, which can lead to further organ damage.
- Growth Retardation in Children: Children who experience severe hemolytic uremic syndrome, especially with prolonged kidney dysfunction, may have growth retardation.
Factors Influencing the Risk and Severity of Complications
- Type of HUS: aHUS tends to have a higher risk of severe and long-term complications, including progression to end-stage renal disease and recurrence, compared to typical STEC-HUS.
- Severity of Initial Presentation: Patients with more severe initial kidney injury, neurological involvement, or significant systemic symptoms are at higher risk for complications.
- Promptness and Appropriateness of Treatment: Early diagnosis and timely initiation of supportive care and specific therapies (especially for aHUS) can help mitigate the risk and severity of complications.
- Underlying Genetic Factors (in aHUS): The specific genetic mutation in aHUS can influence the severity, prognosis, and risk of recurrence.
- Overall Health of the Patient: Pre-existing medical conditions can impact the patient’s ability to tolerate the acute illness and increase the risk of complications.
Prognosis of Hemolytic Uremic Syndrome
The prognosis of hemolytic uremic syndrome varies significantly depending on several factors, most notably the type of hemolytic uremic syndrome (STEC-HUS, aHUS, or secondary HUS), the severity of the initial illness, the promptness and effectiveness of treatment, and the presence of complications.
STEC-HUS (Typical Hemolytic Uremic Syndrome)
Generally, the prognosis for STEC-HUS is relatively good with appropriate supportive care. Most children with STEC-HUS will recover kidney function. The duration of the acute phase, including the need for dialysis, can vary from days to weeks.
The mortality rate in STEC-HUS is generally low in developed countries, typically ranging from 1-5%, primarily due to severe neurological complications or overwhelming systemic illness during the acute phase.
While most patients recover significant kidney function, a proportion (estimated between 10-30%) may develop long-term sequelae, including:
- Chronic Kidney Disease (CKD): Ranging from mild abnormalities like persistent proteinuria or reduced glomerular filtration rate (GFR) to end-stage renal disease requiring dialysis or transplantation. The risk is higher in those with prolonged oliguria or severe initial kidney injury.
- Hypertension: It can persist in some individuals.
Long-term neurological deficits are less common but can occur in those who had severe neurological involvement during the acute phase. Most individuals who recover from STEC-HUS have a good long-term quality of life.
Atypical Hemolytic Uremic Syndrome (aHUS)
Historically, aHUS had a much poorer prognosis compared to STEC-HUS, with a significant risk of progressive kidney failure, need for long-term dialysis, and mortality (up to 25% in the acute phase). Recurrence rates were also high.
The advent of complement inhibitors, particularly eculizumab and ravulizumab, has dramatically improved the prognosis of aHUS. Early diagnosis and treatment with complement inhibitors have significantly reduced the rates of kidney failure and mortality in the acute phase. Many patients can avoid or discontinue dialysis.
Long-term kidney function outcomes are significantly better with complement inhibition, although some patients may still develop CKD, especially if treatment is delayed or if there is significant pre-existing kidney damage.
The risk of recurrence remains a concern, particularly for patients with certain genetic mutations. Long-term prophylactic treatment with complement inhibitors may be necessary in some cases.
Long-term mortality rates have also decreased with the availability of targeted therapies. Early and effective treatment can also improve outcomes in cases with extra-renal manifestations of aHUS.
Secondary Hemolytic Uremic Syndrome
The prognosis of secondary hemolytic uremic syndrome is highly variable and depends largely on the underlying condition that triggered the hemolytic uremic syndrome. If the underlying cause can be effectively treated, the hemolytic uremic syndrome may resolve.
The risk of long-term kidney damage and other complications depends on the severity and duration of the hemolytic uremic syndrome and the impact of the primary illness. For example, hemolytic uremic syndrome associated with aggressive malignancies may have a poorer overall prognosis due to the underlying cancer.
Factors Influencing Prognosis in All Types of Hemolytic Uremic Syndrome
- Age: Infants and very young children may have a higher risk of severe complications.
- Severity at Presentation: Patients with more severe initial kidney injury (prolonged anuria), significant neurological involvement, or severe hypertension tend to have a worse prognosis.
- Promptness of Diagnosis and Treatment: Early recognition of hemolytic uremic syndrome and timely initiation of appropriate supportive care and specific therapies (especially for aHUS) are critical for improving outcomes.
- Presence of Complications: The development of severe complications like prolonged neurological deficits or bowel infarction worsens the prognosis.
- Underlying Genetic Factors (in aHUS): The specific genetic mutation in aHUS can influence disease severity, response to treatment, and risk of recurrence.
- Overall Health: Patients with pre-existing comorbidities may have a poorer prognosis.
Prevention (Primarily for STEC-HUS)
The primary focus of prevention for STEC-HUS revolves around preventing infection with Shiga toxin-producing Escherichia coli (STEC), particularly E. coli O157:H7 and other non-O157 STEC strains.
Food Safety Practices
This is the most critical area for prevention:
- Thorough Cooking of Meat: Ensure that meat, especially ground beef, is cooked to an internal temperature of at least 160°F (71°C). Use a food thermometer to verify. Ground beef should be cooked until it is no longer pink in the center and the juices run clear.
- Avoid Undercooked Meat: Be cautious with rare or undercooked hamburgers and other ground meat products.
- Safe Handling of Raw Meat: Prevent cross-contamination by keeping raw meat separate from ready-to-eat foods. Wash hands, cutting boards, countertops, and utensils thoroughly with hot, soapy water after contact with raw meat.
- Avoid Unpasteurized Milk and Dairy Products: Only consume pasteurized milk, cheese, and other dairy products.
- Drink Pasteurized Juices and Cider: Avoid unpasteurized fruit juices and ciders, as they can be contaminated with STEC.
- Wash Fruits and Vegetables Thoroughly: Wash all raw fruits and vegetables under running water before eating, cutting, or cooking, especially those that will not be cooked. Scrub firm produce like melons and cucumbers.
- Prevent Cross-Contamination in the Kitchen: Use separate cutting boards for raw meats and produce. Wash hands thoroughly before and after handling food.
- Safe Water Sources: Drink water from safe, treated sources. Avoid drinking untreated water from lakes, rivers, or wells.
- Hygiene Practices: Good hygiene can help prevent the spread of STEC.
- Frequent and Thorough Handwashing: Wash hands thoroughly with soap and water for at least 20 seconds, especially after using the toilet, changing diapers, before preparing or eating food, and after contact with animals or their environment.
- Proper Diaper Changing: Follow safe diaper changing practices and wash hands thoroughly afterward.
- Avoid Swallowing Water During Recreational Activities: Be cautious when swimming in lakes, pools, or other recreational waters to avoid swallowing water that may be contaminated.
Public Health Measures
- Surveillance and Outbreak Investigation: Public health agencies play a crucial role in monitoring for STEC infections and investigating outbreaks to identify the source and implement control measures.
- Education and Awareness Campaigns: Public education campaigns are essential to inform the public about the risks of STEC infection and how to prevent it.
- Regulations and Inspections: Government regulations and inspections of food processing facilities and agricultural practices help ensure food safety standards are met.
- Animal Husbandry Practices: Research into and implementation of practices in animal agriculture that reduce STEC carriage in livestock may help reduce the source of contamination.
Frequently Asked Questions (FAQs)
Which antibiotic causes hemolytic uremic syndrome?
While antibiotics are not a direct cause of hemolytic uremic syndrome in the same way that Shiga toxin-producing E. coli (STEC) is, their use in the context of STEC infections is controversial and generally avoided because some evidence suggests they might increase the risk or severity of hemolytic uremic syndrome.
Who is highly susceptible to hemolytic uremic syndrome?
- Young Children: Children under the age of 5 are the most susceptible group, especially to the typical form of hemolytic uremic syndrome (STEC-HUS) that follows diarrheal illness. Their developing immune systems and kidneys may make them more vulnerable to the effects of Shiga toxin.
- Individuals with Weakened Immune Systems (Immunocompromised): People with HIV/AIDS, cancer, those undergoing chemotherapy or taking immunosuppressant drugs (e.g., transplant recipients) have a higher risk of developing hemolytic uremic syndrome if infected with STEC or due to other causes.
- Individuals with Certain Genetic Predispositions: People with specific gene mutations that affect the complement system are highly susceptible to atypical HUS (aHUS). This form of hemolytic uremic syndrome can be triggered by infections or other factors but is primarily driven by dysregulation of the complement system. A family history of hemolytic uremic syndrome may also indicate this susceptibility.
- Older Adults: While hemolytic uremic syndrome is less common in adults, older adults (especially those over 65) who develop hemolytic uremic syndrome tend to have more severe disease and a higher risk of complications and mortality compared to children. This may be due to underlying health conditions and a less robust immune response.
- Individuals with Prolonged Diarrheal Illness: In the context of STEC infection, individuals who experience a longer duration of diarrhea appear to have a higher risk of progressing to hemolytic uremic syndrome.
- Possibly Females: Some studies have suggested a slightly higher incidence of hemolytic uremic syndrome in females, particularly in older children and adolescents, although this is not a consistently strong risk factor across all age groups.
What are the first signs of E. coli infection?
The first signs of an E. coli infection, particularly the types that cause gastrointestinal illness, typically involve the sudden onset of watery diarrhea. This diarrhea can range from mild to severe and is sometimes accompanied by abdominal cramps and pain, which can be quite intense. Some individuals may also experience nausea and vomiting along with these initial digestive symptoms. While a low-grade fever can occur, it is not always present as one of the primary early indicators of an E. coli infection affecting the gut. Symptoms usually manifest within 1 to 10 days after exposure to the bacteria. In hemolytic uremic syndrome, bloody diarrhea can be a clinical manifestation.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Espadinha D, Brady M, Brehony C, Hamilton D, O’Connor L, Cunney R, Cotter S, Carroll A, Garvey P, McNamara E. Case-Control Study of Factors Associated with Hemolytic Uremic Syndrome among Shiga Toxin-Producing Escherichia coli Patients, Ireland, 2017-2020. Emerg Infect Dis. 2025 Apr;31(4):728-740. doi: 10.3201/eid3104.240060. PMID: 40133048; PMCID: PMC11950266.
- Stea ED, Pugliano M, Gualtierotti R, Mazzucato M, Santangelo L, Annicchiarico G, Berardelli A, Bianchi S, Bogliolo L, Chiandotto P, Cirino G, Iaco F, Rosa S, Dentali F, Facchin P, Favalli EG, Fiorin F, Giarratano A, Laterza C, Macrì F, Mancuso M, Padovani A, Pasini A, Scopinaro AM, Sebastiani GD, Sesti G, Susi B, Torsello A, Vezzoni C, Zanlari L, Gesualdo L, Luca A. Multidisciplinary consensus on the diagnosis and management of patients with atypical Hemolytic Uremic Syndrome (complement-mediated TMA): recommendations from Italian scientific societies, patient associations and regulators. Pharmacol Res. 2025 Apr 7:107714. doi: 10.1016/j.phrs.2025.107714. Epub ahead of print. PMID: 40204022.
- Bogdan RG, Anderco P, Ichim C, Cimpean AM, Todor SB, Glaja-Iliescu M, Crainiceanu ZP, Popa ML. Atypical Hemolytic Uremic Syndrome: A Review of Complement Dysregulation, Genetic Susceptibility and Multiorgan Involvement. J Clin Med. 2025 Apr 7;14(7):2527. doi: 10.3390/jcm14072527. PMID: 40217974; PMCID: PMC11989465.
- Vujović A, Sellier-Leclerc AL, Mancuso MC, Boyer O, Awan A, Gargiulo A, Loos S, Fila M, Jankauskiene A, Ariceta G, Kanzelmeyer N, Vidal E, Van Dyck M, Levart TK, Šimánková N, Decramer S, Hofstetter J, Vivarelli M, Sciascia S, van de Kar NCAJ, Schaefer F; ERKNet TMA Working Group. Real-world use of complement inhibitors for haemolytic uraemic syndrome: an analysis of the European Rare Kidney Disease Registry cohort. EClinicalMedicine. 2025 Mar 27;82:103159. doi: 10.1016/j.eclinm.2025.103159. PMID: 40224677; PMCID: PMC11987679.
- Yang L, Liu F, Li X, He L, Gu Y, Sun M, Liu Z, Liu Z. Plasma exchange combined with eculizumab in the management of atypical hemolytic uremic in pediatric patients: A case report. Medicine (Baltimore). 2025 Apr 11;104(15):e42090. doi: 10.1097/MD.0000000000042090. PMID: 40228287; PMCID: PMC11999395.
- Varga P, Biró E, Berkes A, Lakatos E, Szikszay E, Prohászka Z, Szabó T. Use of complement C5-inhibitor eculizumab in patients with infection-associated hemolytic uremic syndrome – a case-series report. BMC Pediatr. 2025 Mar 11;25(1):181. doi: 10.1186/s12887-025-05546-3. PMID: 40065282; PMCID: PMC11895294.