TL;DR
Platelet function disorders disrupt the ability of platelets to clump together (aggregate) and form a proper clot. Platelets are blood cells essential for forming clots and stopping bleeding.
- Inherited: Caused by genetic mutations affecting platelet proteins. Examples include Glanzmann’s disease, Bernard-Soulier syndrome, and storage pool deficiencies.
- Acquired: Occur later in life due to various factors like medications, diseases, or kidney failure. Examples include drug-induced immune thrombocytopenia (DIT), antiplatelet drugs, hyperglobulinemia, myeloproliferative neoplasms (MPN), myelodysplastic syndromes (MDS), and uremia.
Symptoms: Easy bruising, excessive bleeding after minor injuries, prolonged bleeding time during menstruation or surgery, bleeding in the mouth or gastrointestinal tract.
Diagnosis ▾: A combination of clinical history, bleeding time tests, platelet aggregation studies, and potentially genetic testing.
Treatment ▾: There is no cure for most inherited disorders, but management focuses on preventing and controlling bleeding. Acquired disorders often involve addressing the underlying cause. General treatment strategies include:
- Avoiding medications that further impair platelet function.
- Antifibrinolytic medications to prevent clot breakdown.
- Platelet transfusions for severe bleeding episodes.
- Medications to stimulate platelet production or release (limited use).
- Splenectomy (in some severe inherited cases).
- Addressing the underlying cause in acquired disorders.
*Click ▾ for more information
Introduction
Platelet function disorders are a group of conditions that impair the ability of platelets to clump together (aggregate) and form blood clots. Platelets are blood cells that are essential for stopping bleeding by forming plugs at the site of injury. When platelets function abnormally, even minor injuries can lead to excessive bleeding.
The Role of Platelets
Platelets act as the body’s first responders in clotting (hemostasis).
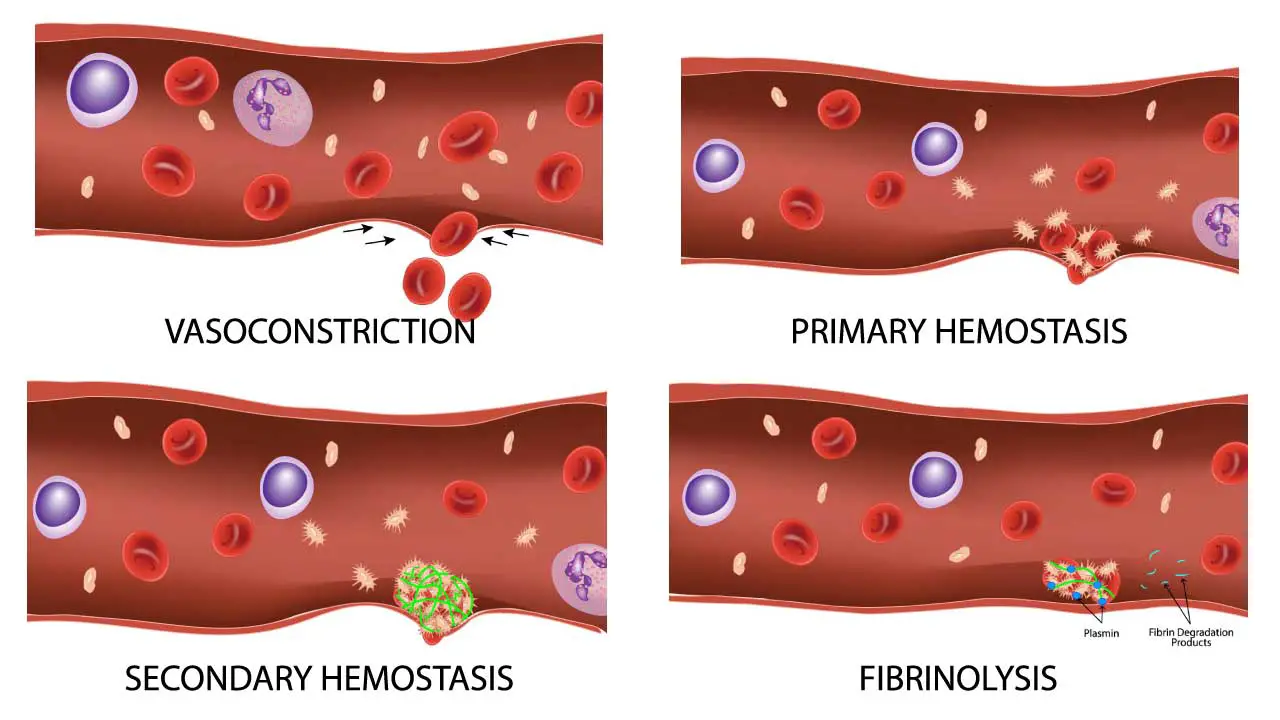
- Injury Detection: When a blood vessel is damaged, platelets are attracted to the exposed collagen fibers in the vessel wall.
- Activation and Adhesion: Platelets become sticky and change shape, sticking to the injured area and each other.
- Plug Formation: By clumping together (aggregation), platelets form a plug that initially seals the breach in the vessel wall.
- Signaling Cascade: Platelets also release chemicals that attract clotting factors, proteins in the blood plasma that work together to form a stronger fibrin mesh, solidifying the clot.
Causes of Platelet Function Disorders
Inherited Disorders
Inherited platelet function disorders are a group of rare conditions caused by genetic mutations that affect platelet proteins or functions. These mutations are typically passed down from parents to their children through autosomal recessive inheritance (both parents must carry the mutated gene) or autosomal dominant inheritance (only one parent needs the mutated gene). These disorders can cause excessive bleeding after minor injuries or even spontaneous bleeding.
Thrombasthenia (Glanzmann’s disease)
- Cause: Mutations in genes encoding glycoprotein (GP) IIb/IIIa complex, the major platelet receptor for fibrinogen (a clotting protein). This complex allows platelets to aggregate and form a clot. It is an autosomal recessive disorder.
- Symptoms: Easy bruising, excessive bleeding after minor injuries (cuts, nosebleeds), prolonged bleeding during menstruation, and bleeding in the mouth or gastrointestinal tract. Symptoms can vary depending on the severity of the mutation.
- Diagnosis: Bleeding time test (prolonged), platelet aggregation studies (reduced aggregation with specific agonists), and genetic testing to confirm the specific mutation.
- Treatment: There is no cure, but management focuses on preventing and controlling bleeding. This may include:
- Avoiding medications that further impair platelet function (e.g., aspirin).
- Antifibrinolytic medications (to prevent breakdown of clots).
- Platelet transfusions during surgery or major bleeding episodes.
- Desmopressin (medication that can stimulate platelet release from storage).
- In some cases, splenectomy (removal of the spleen, which can store platelets) may be considered.
Bernard-Soulier syndrome
- Cause: Mutations in genes encoding the GPIb/IX/V complex, a platelet receptor for von Willebrand factor (vWF), a protein that helps platelets adhere to the injured vessel wall. These giant platelets have abnormal storage and release of granules containing clotting factors.
- Symptoms: Similar to Glanzmann’s disease, including easy bruising, excessive bleeding, and prolonged bleeding time. Additionally, due to the giant platelets, some individuals may experience a higher risk for blood clots in unusual locations (e.g., small blood vessels).
- Diagnosis: Similar to Glanzmann’s disease with the addition of tests to identify the giant platelets and potential abnormalities in vWF binding.
- Treatment: Management is similar to Glanzmann’s disease, focusing on preventing and controlling bleeding. Platelet transfusions may be less effective due to the giant platelet size.
Storage Pool Diseases
- Cause: Storage pool diseases (SPDs) are a specific type of inherited platelet dysfunction disorder affecting the storage granules within platelets. These granules contain essential clotting factors and signaling molecules vital for platelet activation and aggregation. In SPDs, mutations affect the normal functioning of these granules, leading to a deficiency in their contents or impaired release during clotting.
- Symptoms: The severity of symptoms in SPDs can vary depending on the specific type and the degree of the deficiency. Common symptoms include:
- Easy bruising and excessive bleeding after minor injuries (cuts, nosebleeds)
- Prolonged bleeding time during menstruation or surgery
- Bleeding in the mouth or gastrointestinal tract
- In some cases, no noticeable bleeding problems
- Diagnosis:
- Clinical history and physical examination: Focusing on bleeding symptoms and family history.
- Blood tests: Complete blood count (CBC) may show normal platelet count, but platelet function tests will reveal abnormalities in aggregation or secretion.
- Electron microscopy: May reveal abnormal morphology of platelets and their granules.
- Genetic testing: Can confirm the specific type of SPD by identifying the underlying mutation.
- Treatment: There is currently no cure for SPDs. Treatment focuses on managing bleeding episodes and preventing complications
Scott Syndrome
- Cause: Scott syndrome is caused by a genetic mutation in an unknown gene. While the exact protein affected remains unidentified, researchers believe the mutation disrupts a cellular process called “scramblase activity.” Scramblase is thought to be involved in flipping a specific phospholipid (phosphatidylserine) within the platelet membrane. This “flip” is crucial for exposing phosphatidylserine to the outer surface of the platelet, which then serves as a platform for clotting factors to assemble and initiate clot formation.
- Symptoms: Easy bruising and excessive bleeding after minor injuries (cuts, nosebleeds). Prolonged bleeding time during menstruation or surgery. Bleeding in the mouth or gastrointestinal tract
- Diagnosis: Diagnosing Scott syndrome can be challenging due to its rarity and the similarity of symptoms to other bleeding disorders.
- Clinical history and physical examination: Focusing on bleeding symptoms and family history (although inheritance patterns are not fully established).
- Blood tests: Complete blood count (CBC) may show normal platelet count. However, specific tests for platelet function will reveal abnormalities in clotting assays, particularly those that rely on phosphatidylserine exposure. These tests may include:
- Annexin V binding assay: Measures the ability of platelets to expose phosphatidylserine on their surface.
- Platelet aggregation studies: May show reduced aggregation with specific agonists.
- Genetic testing: While not yet a routine diagnostic tool, genetic testing for mutations associated with Scott syndrome is under development.
- Treatment: There is no cure for Scott syndrome. Treatment focuses on managing bleeding episodes and preventing complications.
Diagnosis of all inherited platelet function disorders typically involves a combination of clinical history, bleeding time tests, platelet aggregation studies, and potentially genetic testing to confirm the specific mutation.
Treatment for inherited platelet function disorders is primarily supportive and focuses on managing bleeding episodes. Gene therapy is a potential future treatment approach, but is still under investigation.
Acquired Disorders
Unlike inherited disorders, acquired causes of platelet dysfunction arise later in life due to various factors.
Antiplatelet Drugs
- Cause: These medications (e.g., aspirin, clopidogrel) are used to prevent blood clots by inhibiting platelet aggregation. This is a desired effect for conditions like heart disease or stroke, but it also increases the risk of bleeding.
- Symptoms: Increased risk of bleeding, especially during surgery, injury, or even routine activities like brushing teeth.
- Diagnosis: Based on the patient’s medical history and medication use. No specific test for the antiplatelet effect, but bleeding time may be prolonged.
- Treatment: There is no way to completely reverse the antiplatelet effect, but management focuses on:
- Balancing the risk of bleeding with the need to prevent blood clots.
- Minimally invasive procedures when possible.
- In some cases, temporary discontinuation of the medication before surgery may be considered under close medical supervision.
Hyperglobulinemia
- Cause: Elevated levels of abnormal proteins (globulins) in the blood can interfere with platelet function by coating them or hindering their interaction with other clotting factors. This can occur in various conditions like:
- Autoimmune diseases (e.g., lupus)
- Chronic infections
- Liver diseases (e.g., cirrhosis)
- Symptoms: Variable depending on the underlying cause, but may include easy bruising, bleeding from gums or nosebleeds, and potential for internal bleeding.
- Diagnosis: Based on clinical presentation, blood tests to identify the underlying condition and measure globulin levels, and potentially tests for specific platelet function abnormalities.
- Treatment: Depends on the underlying cause. May involve treating the autoimmune disease, managing the infection, or addressing the liver dysfunction. In some cases, medications or plasma exchange can be used to reduce globulin levels. Platelet transfusions may be needed for severe bleeding.
Myeloproliferative Neoplasms (MPN) & Myelodysplastic Syndromes (MDS)
- Cause: These are bone marrow disorders that can affect platelet production and function:
- MPN: Abnormal increase in blood cell production, including platelets. However, these platelets may be dysfunctional.
- MDS: Abnormal development of blood cells, leading to a decrease in the number or function of platelets.
- Symptoms: Variable depending on the specific disorder, but may include easy bruising, bleeding, fatigue, and potential for blood clots (paradoxical in some MPNs).
- Diagnosis: Blood tests to assess blood cell counts and morphology (appearance), bone marrow biopsy to evaluate cell production.
- Treatment: Depends on the specific MPN or MDS type. May involve medications to regulate blood cell production (e.g., hydroxyurea), chemotherapy, or even stem cell transplantation in severe cases. Platelet transfusions may be needed for severe bleeding.
Uremia
- Cause: Kidney failure leads to a buildup of waste products in the blood, which can impair platelet function and hinder their ability to aggregate.
- Symptoms: Easy bruising, bleeding, and may be associated with other uremic symptoms (e.g., nausea, fatigue).
- Diagnosis: Based on clinical presentation, blood tests to assess kidney function and identify waste product buildup. Platelet function tests may also be performed.
- Treatment: Focuses on managing the underlying kidney disease. Dialysis (hemodialysis or peritoneal dialysis) helps remove waste products from the blood. In some cases, medications or plasma exchange can be used to improve platelet function. Platelet transfusions may be needed for severe bleeding.
Diagnosis of Platelet Function Disorders
Platelet function disorders can manifest through various bleeding symptoms. However, a normal platelet count doesn’t necessarily rule them out. To confirm a suspected platelet dysfunction and determine the underlying cause, there are a combination of tests that evaluate different aspects of platelet function.
Bleeding Time Tests
- Template bleeding time (TBT): This standardized test measures the time it takes for bleeding to stop from a standardized small cut on the forearm. A prolonged TBT suggests a potential platelet dysfunction.
- Ivy bleeding time: This older test, less commonly used now, involves measuring bleeding time from a standardized venipuncture site.
Platelet Aggregation Studies
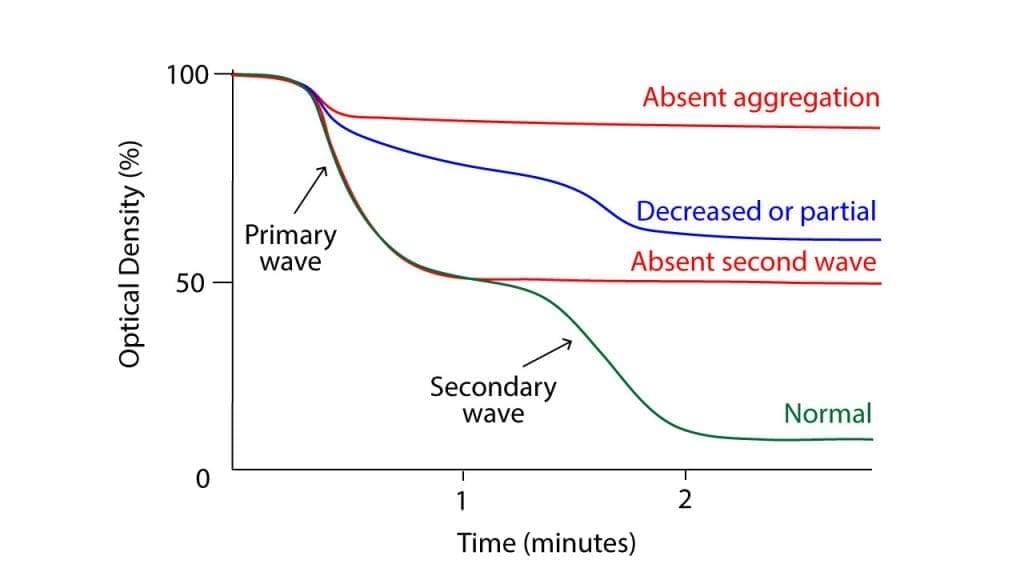
This group of tests assesses the ability of platelets to clump together (aggregate) when stimulated by various substances (agonists) that mimic their activation during clotting. Different agonists target specific platelet receptors, and the resulting aggregation pattern can help identify the type of dysfunction.
- Light transmission aggregometry (LTA): This traditional method measures changes in light transmission through platelet-rich plasma as platelets aggregate, providing an indirect measure of aggregation.
- Multi-electrode aggregometry (MEA): A newer technology that measures electrical impedance changes associated with platelet aggregation, offering a more sensitive and detailed analysis.
Other Tests
- Flow cytometry: This technique uses lasers to analyze the presence and function of specific proteins on the platelet surface. It can identify inherited platelet disorders associated with abnormalities in these proteins.
- Electron microscopy: This high-resolution imaging technique can reveal abnormal morphology of platelets and their storage granules, which may be helpful in diagnosing storage pool diseases.
- Genetic testing: While not routinely used for all disorders, genetic tests can identify specific mutations responsible for some inherited platelet function disorders.
Limitations of Testing
It’s important to note that no single test is perfect for diagnosing all platelet function disorders. Some tests may have limitations in sensitivity or specificity. Additionally, some acquired causes of platelet dysfunction, such as uremia or medications, may not show abnormalities on all tests.
In conclusion, a comprehensive approach using a combination of clinical evaluation, bleeding time tests, platelet aggregation studies, and other specialized tests, along with expert interpretation, is crucial for accurate diagnosis and management of platelet function disorders.
Management of Platelet Function Disorders
There is no one-size-fits-all treatment for platelet function disorders. The specific approach depends heavily on the underlying cause of the dysfunction.
Inherited Platelet Function Disorders
- No cure exists, but treatment focuses on managing bleeding episodes and preventing complications.
- Avoiding medications that further impair platelet function: This includes medications like aspirin, ibuprofen, or other nonsteroidal anti-inflammatory drugs (NSAIDs) that can inhibit platelet aggregation.
- Antifibrinolytic medications: These medications (e.g., tranexamic acid, epsilon aminocaproic acid) help prevent the breakdown of existing clots, minimizing bleeding.
- Platelet transfusions: During surgery, major injuries, or severe bleeding episodes, platelet transfusions can increase the number of functional platelets and support clotting.
- Desmopressin (DDAVP): This medication can stimulate the release of platelets from storage in some cases, particularly for patients with storage pool deficiencies.
- Splenectomy (removal of the spleen): In some severe cases, especially with inherited disorders like Glanzmann’s thrombasthenia, splenectomy can be considered. The spleen can trap and destroy platelets, and its removal may improve platelet availability.
- Emerging therapies: Gene therapy is a potential future treatment option for some inherited disorders, but it’s still under investigation.
Acquired Platelet Function Disorders
The primary focus is on addressing the underlying cause.
- Antiplatelet drugs: For patients who require these medications for conditions like heart disease or stroke, managing the bleeding risk becomes a balancing act. This may involve using the lowest effective dose, minimizing invasive procedures, and considering temporary discontinuation before surgery under close medical supervision.
- Hyperglobulinemia: Treatment depends on the underlying cause. Managing autoimmune diseases, controlling chronic infections, or addressing liver dysfunction can improve overall health and potentially reduce the impact on platelet function. In some cases, medications or plasma exchange may be used to directly reduce globulin levels.
- MPN & MDS: Treatment for these bone marrow disorders varies depending on the specific type. Medications like hydroxyurea may help regulate blood cell production. In severe cases, chemotherapy or even stem cell transplantation might be necessary.
- Uremia: Treatment focuses on managing kidney failure. Dialysis (hemodialysis or peritoneal dialysis) helps remove waste products from the blood, which can improve platelet function over time. Additionally, medications or plasma exchange may be used in specific situations.
General Supportive Measures
- Avoiding activities that increase bleeding risk: This may include contact sports or using sharp objects like razors.
- Maintaining good oral hygiene: To minimize bleeding from the gums.
- Regular follow-up with a healthcare professional: To monitor the condition and adjust treatment plans as needed.
Overall, treatment for platelet function disorders aims to:
- Reduce bleeding risk and manage bleeding episodes.
- Improve platelet function, if possible.
- Address the underlying cause, if applicable.
- Maintain a good quality of life for the patient.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.