Procedure At A Glance
The purpose of Rh typing is to determine the presence or absence of the RhD antigen on an individual’s red blood cells to prevent transfusion reactions and hemolytic disease of the newborn (HDN).
- Label tubes.
- Add a drop of a 2-5% patient red cell suspension to the appropriately labeled tubes.
- Add one drop of Anti-D antiserum to the Anti-D labeled tube and one drop of control reagent to the control tube.
- Gently mix the contents of each tube.
- Incubate the tubes at the recommended temperature.
- Centrifuge the tubes.
- Gently resuspend the red cell buttons and observe for agglutination (clumping of red cells).
Introduction
Apart from the ABO blood group system, the Rh blood group system is also important because anti-RhD can cause hemolytic transfusion reactions (highly immunogenic) and are normally IgG. Anti-RhD is able to cross the placenta and lead to hemolytic disease of the newborn (HDN). The Rh blood group system was discovered in 1939 by Karl Landsteiner and Alexander Wiener.
What is the Rh Blood Group System?
The Rh blood group system consists of two homologous transmembrane proteins, both encoded by genes on chromosome 1, that span the red blood cell plasma membrane 12 times. They appear to be used for the transport of carbon dioxide and/or ammonia across the plasma membrane.
The Rh blood group locus is composed of 2 related structural genes, RhD and RhCE, which encode the membrane proteins that carry the D, Cc and Ee, antigens.
The RhD gene may be either present or absent, giving the Rh D+ or Rh D- phenotype, respectively. Alternative RNA splicing from the RhCE gene generates 2 proteins, which encode the C, c, E or e antigens.
Rh blood group antibodies rarely occur naturally; most are immune. Anti-D is responsible for most of the clinical problems associated with the system and a simple Rh typing of subjects into Rh D+ and Rh D- using anti-D is sufficient for routine clinical purposes. Anti-C, anti-c, anti-E and anti-e are occasionally seen and may cause both transfusion reactions and hemolytic disease of the newborn. Anti-d does not exist.
While routine ABO Rh test often focuses on the RhD antigen, also known as the “D” positive or negative status, there’s a whole world of Rh typing waiting to be explored. Beyond D, several other Rh blood group antigens like C, c, E, and e play crucial roles in blood compatibility. Antibodies against these antigens can cause hemolytic reactions if incompatible blood is transfused. Luckily, commercially available reagents like anti-C, anti-c, anti-E, and anti-e can help identify these additional Rh phenotypes through Rh typing.
Rh typing can be performed using various techniques, including tube agglutination tests and gel card tests. While tube tests were the traditional method, gel cards have become increasingly popular due to their ease of use, speed, and improved sensitivity.
Principle of Rh Typing
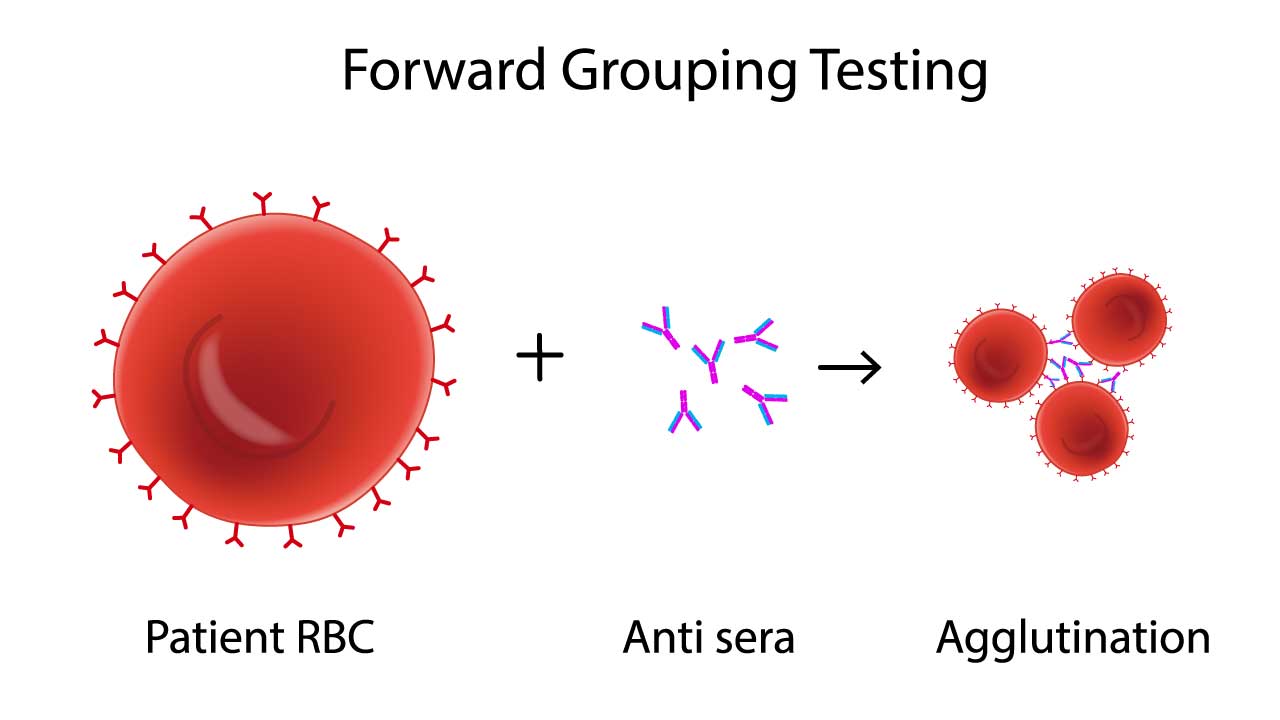
At the heart of the Rh typing procedure lies the concept of antigen-antibody interaction, similar to ABO RhD blood group typing. Rh proteins, like D, C, E, and e, adorn the surface of red blood cells, acting as antigens. If the body lacks a specific antigen, say D, it may develop antibodies against it (anti-D). Transfusing incompatible blood bearing that antigen triggers a violent immune response, potentially leading to life-threatening hemolytic reactions.
Red blood cells in Rh typing are first suspended in a solution containing antisera, each targeting a specific Rh antigen (e.g., anti-D, anti-C, anti-E). If a cell possesses the specific antigen, it binds to the corresponding antibody, forming visible clumps called agglutination. The absence of agglutination indicates a lack of the antigen
If a potential incompatibility is detected, red blood cells are subjected to individual tests with each antisera. This detailed analysis confirms the presence or absence of specific Rh antigens, allowing for a precise determination of the individual’s Rh phenotype.
Materials
- Polypropylene/glass tubes 10 mm x 75 mm
- Serofuge
- Centrifuge
- Pasteur pipettes
- Commercially prepared anti-D, -C, -E, -c and -c sera
- Patient’s EDTA whole blood
- Normal saline
Protocol
- Label each test tube with the corresponding antibody (Anti-D, Anti-C, etc.). Include a control tube with no antibody.
- Using a Pasteur pipette, add one drop of blood into each test tube.
- Add two drops of the appropriate antisera to each respective tube.
- Gently flick or rotate the tubes to mix the blood and reagents thoroughly.
- Place the tubes in a 37°C incubator for 30 minutes to allow for optimal antigen-antibody reactions.
- After incubation, centrifuge the tubes for 1 minute at 1000 RPM.
- Gently resuspend the cell button by tapping the tubes.
- Examine each tube under a light source to look for visible clumping or agglutination of red blood cells.
Interpretation
Positive results Rh typing are indicated by agglutination of tested blood. A negative result is observed when there is a clear supernatant after resuspension in the red cell agglutination test.
- Agglutination in a tube: Indicates the presence of the corresponding antigen on the red blood cells in Rh typing.
- No agglutination: Indicates the absence of the corresponding antigen.
- Control tube: Should show no agglutination, confirming the validity of the test.
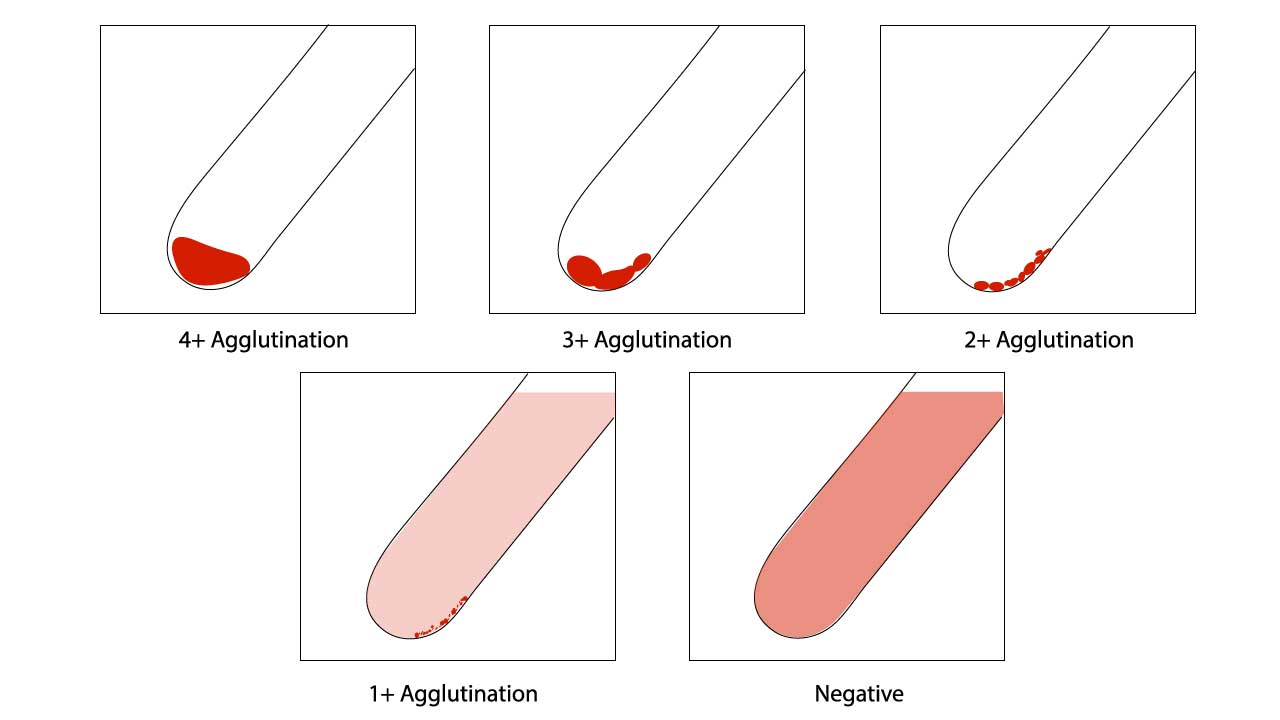
Assessment of red cell agglutination test grading
| Symbol | Agglutination score | Description |
| 4+ / Complete (C) | 12 | Macroscopically visible cell button with a clear supernatant. |
| 3+ | 10 | Macroscopically visible large clumps of cell button with a clear supernatant. |
| 2+ | 8 | Macroscopically visible small clumps of cell button with a clear supernatant. |
| 1+ | 5 | Just macroscopically visible fine granular clumps of the cell button and the supernatant is turbid and reddish. |
| or weak (w) | 3 | Only microscopically visible fine granules of the cell button and the supernatant is turbid. |
| 0 | 0 | No agglutination seen. The supernatant is clear and reddish in color. |
| MF (mixed field) | MF | A mixture of agglutinated and unagglutinated red cells seen. |
| H | Complete hemolysis of the patient sample. The supernatant is grossly red with no evidence of red cells. |
Troubleshooting
Even with meticulous technique, discrepancies or unexpected results can occur in Rh typing. Effective troubleshooting is crucial to ensure accurate patient blood typing and transfusion safety.
Weak or Questionable Agglutination (Weak Positive / Grade 1+)
This is a common challenge in Rh typing that can lead to false negatives if misinterpreted.
Causes
- Weak D (Du) Phenotype: Some individuals express a weaker form of the D antigen that may not react strongly in immediate spin (IS) or room temperature testing in Rh typing. These often require further testing using the Indirect Antiglobulin Test (IAT) phase (37°C incubation followed by Anti-Human Globulin/Coombs reagent).
- Expired or Deteriorated Reagents: Anti-D antisera can lose potency over time or due to improper storage.
- Improper Cell Suspension: A cell suspension that is too dilute can lead to weak reactions in Rh typing.
- Insufficient Incubation Time or Temperature: If the reagent requires a specific incubation time or temperature (e.g., 37°C), deviations can affect reaction strength.
- Under-centrifugation: Not optimal centrifugal force can lead to loosely packed cell buttons and weak agglutination.
- Over-shaking/Vigorous Resuspension: Agglutinates, especially weak ones, can be broken apart by excessive agitation.
Solution
- Perform a Weak D (Du) test (if not already part of your protocol for all D-negative results). This typically involves incubating the negative tubes at 37°C, washing, and adding Anti-Human Globulin.
- Repeat the Rh typing test with a freshly prepared cell suspension of the correct concentration.
- Check reagent expiration dates and storage conditions. Use a new vial if suspicious.
- Ensure proper incubation time and temperature.
- Verify centrifuge calibration and ensure correct speed and duration.
- Gently resuspend the cell button.
False Positive Results (Agglutination in the Rh Control Tube or Unexpected D Positivity)
A positive Rh control invalidates the Rh typing result.
Causes
- Autoagglutinins/Cold Agglutinins: Patient’s red cells may be coated with antibodies that cause them to spontaneously agglutinate, even without the presence of anti-D reagent. This often causes agglutination in all tubes, including the control.
- Rouleaux Formation: Abnormal plasma proteins (e.g., in multiple myeloma) can cause red cells to stack like coins, which can be mistaken for agglutination. The control tube in this Rh typing will also show rouleaux. Rouleaux usually disperses with saline addition.
- Bacterial Contamination: Contaminated samples or reagents can lead to non-specific agglutination.
- Heavy Cell Suspension: An overly concentrated red cell suspension can sometimes lead to pseudoagglutination or difficulty distinguishing true agglutination.
- Dirty Glassware: Residue in tubes can cause non-specific reactions in Rh typing.
- High-Protein Reagent Issues: If using high-protein anti-D reagents, a positive control indicates non-specific agglutination.
- Fibrin Clots: Inadequately clotted or hemolyzed samples can have fibrin strands that trap red cells, mimicking agglutination.
Solution
- Wash Red Cells: Repeatedly washing the patient’s red cells with saline (at least 3-4 times) can often remove autoagglutinins or excess proteins causing rouleaux. Re-test with washed cells.
- Microscopic Examination: Examine agglutinates microscopically to differentiate true agglutination from rouleaux (rouleaux appears as coin-stacks, true agglutination is irregular clumping). Adding a drop of saline can help disperse rouleaux.
- Use Saline-Diluted Reagents/Monoclonal Anti-D: If using high-protein anti-D, switch to a low-protein or monoclonal anti-D reagent (which usually come with a diluent or saline control) that is less prone to non-specific reactions.
- Check Specimen Quality: Ensure the sample is fresh, properly collected, and not hemolyzed or clotted before Rh typing.
- Cleanliness: Use clean, dry test tubes.
False Negative Results (Missing Expected D Positivity or Unexpected D Negativity)
This is particularly dangerous in transfusion medicine as it can lead to alloimmunization.
Causes
- Failure to Add Reagent/Cells: Simple omission of anti-D or red cell suspension in the Rh typing.
- Weak D (Du) Phenotype (as above): If the lab procedure doesn’t include an IAT phase for all D-negative samples, a Weak D might be missed.
- Reagent Deterioration/Improper Storage: Loss of anti-D potency.
- Over-centrifugation: Can pack cells so tightly that agglutination is difficult to dislodge and observe.
- Vigorous Shaking/Resuspension: Disruption of weak agglutinates.
- Immunoglobulin-Coated Cells (in vivo): If the patient’s red cells are already coated with antibodies in vivo (e.g., in Autoimmune Hemolytic Anemia), the anti-D may not be able to bind effectively or agglutination may be masked. A positive DAT would indicate this.
- Incorrect Cell Suspension Concentration: Too strong a suspension can sometimes lead to a prozone effect or simply make weak agglutination harder to discern.
Solution
- Review Procedure: Re-check all steps to ensure no omission of reagents or cells in the Rh typing.
- Perform Weak D Test: Essential for all initial Rh-negative results.
- Check Reagents: Verify integrity (expiration, storage) of all reagents used in the Rh typing.
- Optimal Centrifugation: Ensure correct speed and time.
- Gentle Resuspension: Re-examine tube carefully.
- Perform a Direct Antiglobulin Test (DAT): If the patient’s cells are DAT positive, further investigation is needed as the Rh typing may be unreliable due to existing antibody coating.
Discrepancies Between Current and Previous Results
Causes
- Transfusion of Rh-Positive Blood to an Rh-Negative Patient: Can cause mixed-field agglutination.
- Recent Transfusion: The presence of transfused cells with a different Rh type.
- Bone Marrow Transplant: Patient’s Rh type may change to that of the donor.
- Clerical Errors: Mislabeling, incorrect data entry, or patient identification mix-ups.
- Antigen Loss due to Disease: Rare conditions (e.g., some leukemias) can lead to weakened or absent Rh antigen expression.
Solution
- Verify Patient Identification: Critical first step.
- Check Patient History: Review recent transfusions, pregnancies, or medical conditions.
- Collect a New Specimen: Rule out pre-analytical errors.
- Perform Molecular Testing: For complex cases, molecular RHD genotyping can definitively determine the RhD status, especially for D variants.
General Troubleshooting Principles
- Follow Manufacturer’s Instructions: Always adhere strictly to the reagent manufacturer’s guidelines for incubation, centrifugation, and reading results when performing Rh typing.
- Quality Control (QC): Ensure daily QC checks for all reagents are within acceptable limits.
- Documentation: Meticulously record all results, observations, and troubleshooting steps in the Rh typing.
- Training and Competency: Ensure personnel performing the Rh typing tests are properly trained and regularly assessed for competency in visual interpretation of agglutination.
- Consultation: For persistent or complex discrepancies in the Rh typing, consult with a more experienced technologist, supervisor, or a reference laboratory.
Frequently Asked Questions (FAQs)
What does type A Rh positive mean?
“Type A Rh positive” (often written as A+) means that an individual’s red blood cells have two key characteristics:
- A Antigens: Their red blood cells carry the “A” antigen on their surface. This is what classifies them as blood type A within the ABO blood group system.
- RhD Antigen (Rh Factor): Their red blood cells also have the RhD protein (also known as the Rh factor) on their surface. The presence of this protein makes them “Rh positive.”
In simple terms, if you have A+ blood, you have both the A antigen and the RhD antigen present on your red blood cells. This is one of the most common blood types.
What happens if you are Rh positive?
If you are Rh positive from Rh typing, it means your red blood cells carry the RhD protein on their surface. For the vast majority of people, being Rh positive has no direct impact on their personal health. The primary significance of Rh positivity arises in specific medical contexts: for blood transfusions, it means you can safely receive both Rh-positive and Rh-negative blood, as your body will not produce antibodies against the RhD protein.
However, the most critical consideration for Rh-positive status relates to pregnancy when the mother is Rh-negative; in such cases, if the baby inherits Rh-positive blood, there is a risk of Rh incompatibility, where the mother’s immune system could develop antibodies against the baby’s Rh-positive red blood cells, potentially causing complications like hemolytic disease of the newborn (HDN) in subsequent pregnancies, though this is largely preventable with proper prenatal care and Rh immune globulin injections.
Can Rh positive give blood to anyone?
No, being Rh positive does not mean you can give blood to anyone. While Rh-positive individuals can receive both Rh-positive and Rh-negative blood, their blood can generally only be transfused to other Rh-positive recipients. This is because Rh-negative individuals, if exposed to Rh-positive blood, can develop antibodies against the RhD protein, which could lead to severe transfusion reactions in the future. The universal red cell donor is O negative, as these red blood cells lack both A/B antigens and the RhD antigen, making them least likely to cause an immune reaction.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- American Association of Blood Banks (AABB). Technical Manual, 21st Edition, 2023.
- Dean L. Blood Groups and Red Cell Antigens [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2005.
- Harmening DM. Modern Blood Banking & Transfusion Practices (FA Davis Company). 2018.
- Li HY, Guo K. Blood Group Testing. Front Med (Lausanne). 2022 Feb 11;9:827619. doi: 10.3389/fmed.2022.827619. PMID: 35223922; PMCID: PMC8873177.
- Rosenkrans D, Zubair M, Doyal A. Rh Blood Group System. [Updated 2023 Aug 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK594252/
- Dean L. Blood Groups and Red Cell Antigens [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2005. Chapter 7, The Rh blood group. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2269/