TL;DR
The Rh blood group system is a classification of blood types based on the presence or absence of certain proteins (antigens) on the surface of red blood cells. Rh incompatibility can lead to serious complications.
Antigen System: Rh antigens on red blood cells come in various combinations, with the D antigen being the most significant.
Rh-Positive vs. Rh-Negative ▾: Individuals are classified as Rh-positive blood type if they have the D antigen and Rh-negative blood type if they lack it.
Transfusion Risks ▾: Rh incompatibility occurs when Rh-negative blood type individuals receive Rh-positive blood, leading to potentially life-threatening hemolytic transfusion reactions.
Pregnancy Concerns ▾: In Rh-negative pregnancies with Rh-positive fetuses (hemolytic disease of the newborn), the mother’s immune system can become sensitized to the D antigen, posing a risk for Rh disease in the baby (erythroblastosis fetalis) with complications like anemia and jaundice.
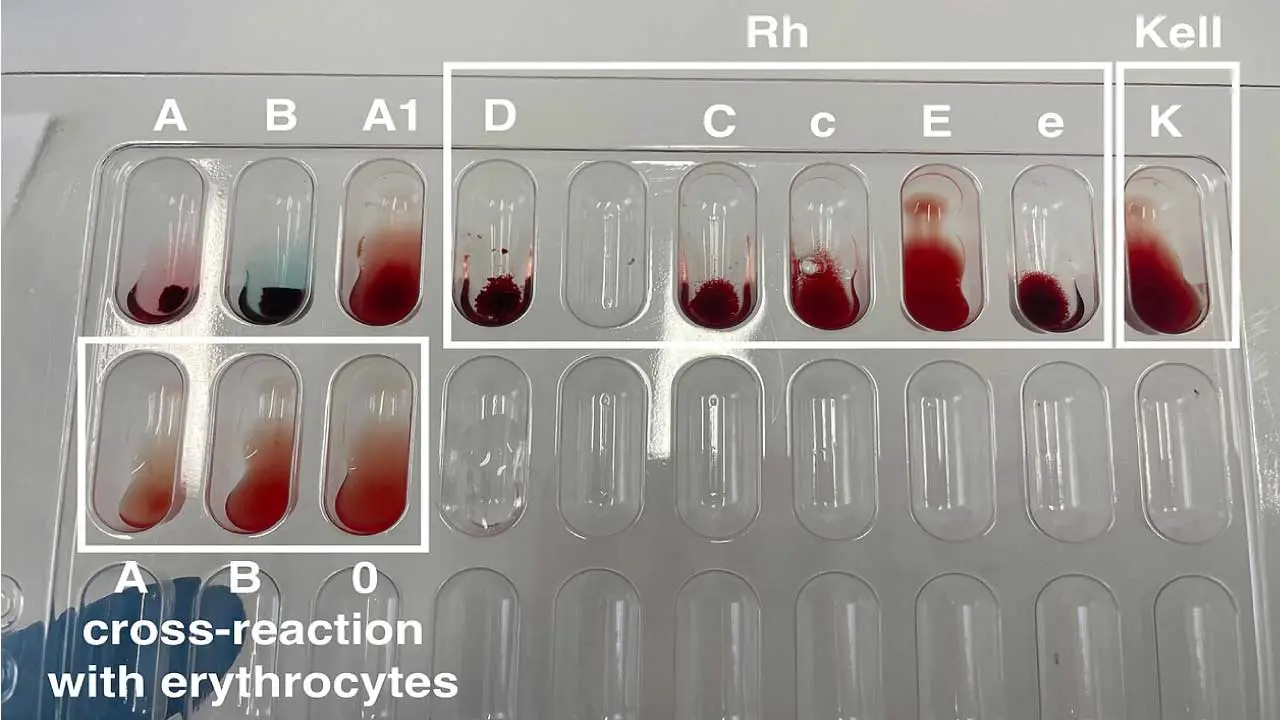
Techniques for Detection ▾: Agglutination tests (tube or solid-phase) with anti-Rh antibodies are used for Rh blood typing. The Indirect Antiglobulin Test (IAT) helps detect weak D variants.
*Click ▾ for more information
Introduction
The Rh blood group system, second only to ABO in importance, classifies people based on antigens on their red blood cells. The most crucial antigen in this group is, RhD, which commonly determines the Rh positive or Rh negative blood type. If a person is Rh negative blood type and is exposed to Rh positive blood (through transfusion or pregnancy), the body can develop antibodies that attack these “foreign” cells, leading to complications like hemolytic transfusion reactions or hemolytic fetal disease of the newborns.
While the Rh system began with the discovery of the D antigen in 1939, it has expanded to encompass over 50 related antigens. This article will focus on the five most important ones – D, C, E, c, and e – and the antibodies they generate, as these are the ones most relevant to transfusion reactions in clinical settings.
History of the Rh Blood Group System
1. Early Observations (1930s)
Prior to the 1940s, blood typing relied solely on the ABO system. Researchers observed cases of unexpected hemolytic transfusion reactions (red blood cell destruction) even when ABO compatibility was confirmed. Dr. Landsteiner and colleagues were investigating a woman experiencing a severe reaction after a second blood transfusion.
2. Discovery through Rhesus Monkeys (1940)
Landsteiner and Wiener injected red blood cells (RBCs) from the woman into rabbits and guinea pigs. These animals developed antibodies that agglutinated (clumped) the woman’s RBCs. Curiously, the antibodies also agglutinated RBCs from about 85% of other people tested. Misconception: Landsteiner and Wiener mistakenly believed the antibodies reacted with an antigen present in both the woman’s RBCs and those of the rhesus monkey (Macaca mulatta), thus naming the system “Rh.”
3. Unraveling the Truth (Later 1940s)
Further research revealed the antigen in human RBCs was distinct from that in rhesus monkeys. The human antigen responsible for the strongest reaction was later designated “RhD.” The name “Rh” stuck, even though the connection to rhesus monkeys was incorrect. The “LW” antigen, originally thought to be the human Rh antigen, was named in honor of Landsteiner and Wiener.
Biochemical Composition
Two closely related genes, RHD and RHCE, located right next to each other on chromosome 1, are responsible for Rh proteins. The RHD gene codes for the RhD protein, carrying the crucial D antigen. The RHCE gene, on the other hand, is a bit more versatile. Depending on its specific version (allele), it can code for proteins with either the C and e antigens or the C and c antigens. This explains the different Rh phenotypes (combinations of antigens) beyond just Rh positive or Rh negative blood type.
The Rh system consists of two homologous transmembrane proteins, both encoded by genes on chromosome 1 with 416 amino acids, that span the red cell plasma membrane 12 times. Unlike most blood group proteins, Rh proteins lack carbohydrate residues and are exclusive to red blood cells as antigens.
Functions of the Rh antigens include:
1. Transmembrane Proteins
Rh proteins are embedded within the membrane of red blood cells, spanning the entire phospholipid bilayer. This positioning allows them to interact with the external environment (blood plasma) and the internal environment of the red blood cell.
2. Possible Ion Channels
Recent research suggests Rh proteins might function as gas channels rather than traditional transporters. They could facilitate the passage of essential gases like:
- Carbon Dioxide (CO2): This aligns with their location on red blood cells, crucial for CO2 removal from tissues to the lungs.
- Ammonia (NH3): This function is still under debate in mammals, but their close relatives in bacteria transport ammonia.
The exact mechanism of Rh protein function is still being unraveled. While the gas channel theory is gaining traction, some evidence suggests they might also have a role in maintaining the shape and flexibility of red blood cells.
Genetics of the Rh Blood Group System
The Rh blood group system is controlled by two closely linked genes on chromosome 1: RhD and RhCE. These genes encode the membrane proteins that carry the D, Cc and Ee antigen. The RhD gene determines Rh positive (Rh D+) or negative (Rh D-) status – if present, it creates the D antigen. The RhD gene follows the autosomal recessive inheritance pattern. Individuals who are homozygous dominant (DD) or heterozygous (Dd) are Rh+ phenotype or have Rh D positive blood type. The lower case d denotes the absence of the gene and not a variant. Those who are homozygous recessive (dd) are Rh negative blood type (Rh-) which means they do not have the Rh D antigens.
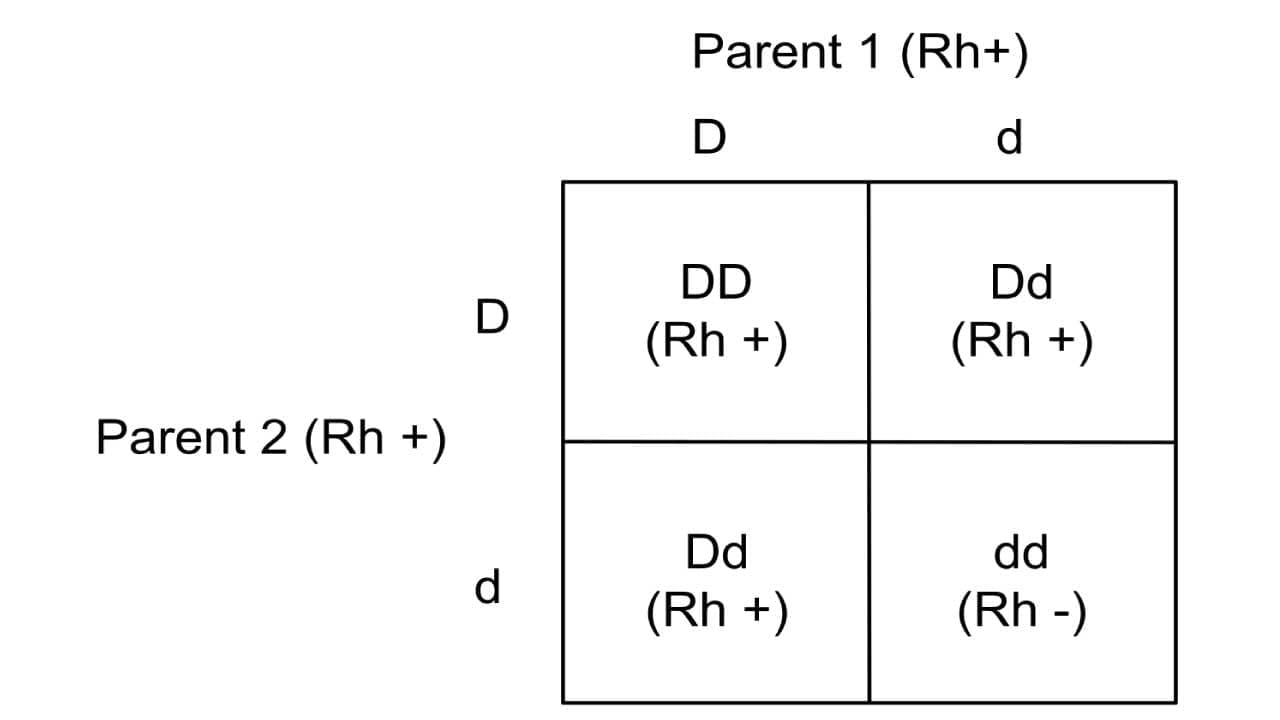
The RHCE gene is more complex. Different alleles of this gene produce either the C and E antigens, or the C and e antigens, through a process called alternative RNA splicing. This explains the variety of Rh phenotypes beyond just D positive or negative.
Gene Loci
- RhD: This gene sits on chromosome 1 and dictates the presence or absence of the D antigen.
- Presence: A functional RhD gene leads to the production of the RhD protein, resulting in an Rh-positive individual.
- Absence: In some cases, the RhD gene is entirely deleted or non-functional, leading to the absence of the D antigen and an Rh-negative phenotype.
- RHCE: Located right next to RhD gene. It has different versions (alleles) that determine the expression of C, E, c, and e antigens:
- C & E allele: This version codes for a protein carrying both the C and E antigens.
- C & e allele: This version codes for a protein with C and c antigens.
Current Genetic Theory of Rh Antigens
The current theory suggests that a gene duplication event in our evolutionary history gave rise to the RHD and RHCE genes. These genes share a high degree of similarity, explaining why some alleles of RHCE can produce proteins with antigens resembling the D antigen (weak D variants). Additionally, various mutations within these genes can lead to the formation of new Rh antigens, contributing to the vast diversity observed in the Rh system.
Fisher-Race vs. Wiener Theory: A Historical Debate
Early attempts to explain Rh antigen inheritance involved two prominent theories.
- Fisher-Race Theory: Proposed that multiple closely linked genes on a single chromosome determine Rh phenotypes. It focused on three main genes (R0, R1, and R2) responsible for various antigen combinations.
- Wiener Theory: Suggested a single gene locus with multiple alleles controlling Rh antigen expression. This theory offered a simpler explanation but faced challenges in explaining certain Rh phenotypes.
Rh Nomenclature
The Rh blood group is complex because of the number of antigens that have been reported. Two commonly used nomenclature systems were proposed before the main antigens were discovered.
Wiener Nomenclature
The Wiener system is favored for spoken communication due to its straightforward approach. Originally based on the idea of a single “agglutinogen” molecule carrying multiple Rh antigens, it uses letters to represent antigen presence or absence.
- D antigen
- Present: Represented by “R” (uppercase)
- Absent: Represented by “r” (lowercase)
- C, c, E, and e antigens
- Carried with D: These are indicated by subscripts after “R”:
- R₀ (R sub zero): Represents c and e antigens together with D.
- R₁ (R sub one): Represents C and e antigens together with D.
- R₂ (R sub two): Represents c and E antigens with D.
- RZ (R sub z): Represents both C and E are present with D.
- Carried without D: These are indicated by superscripts after “r”:
- r’ (r prime): Represents C and e together without D.
- r” (r double prime): Represents c and E without D.
- ry (r superscript y): Represents where both C and E are present without D.
- r (No superscript): Simply “r” signifies the absence of both C and E antigens (along with the absence of D).
- Carried with D: These are indicated by subscripts after “R”:
Fisher-Race Nomenclature
Developed based on the idea of three linked genes (D, C/c, and E/e), the Fisher-Race system uses letters to represent antigens.
- D antigen:
- Present: D (uppercase)
- Absent: Although the system originally included a lowercase “d” for the absence of D, it’s often omitted to avoid confusion because there’s no actual “d” antigen.
- C, c, E, and e antigens: The Fisher-Race system assumes these four antigens come in pairs (C with either c or E with either e), but on the same gene (the RhCE gene, not three separate genes as originally thought).
- This gene can have different combinations of these antigens, resulting in eight possible “haplotypes” (inherited sets of genes from one parent).
Fisher-Race uses a three-letter code (DCE) to represent these haplotypes. Each letter is uppercase (D, C, E) if the corresponding antigen is present and lowercase (d, c, e) if it’s absent.
For example:
- DCE: This represents a complete haplotype with all three antigens (D, C, and E).
Comparison of Wiener and Fisher-Race Nomenclature
| Gene (Wiener) | Antigens (Fisher-Race) |
| R₀ | cDe |
| R₁ | CDe |
| R₂ | cDE |
| RZ | CDE |
| r’ | Ce |
| r” | cE |
| ry | CE |
| r | ce |
3. Rosenfield/Alphanumeric Terminology:
The Rosenfield system takes a unique approach, assigning numbers to each Rh antigen based on its biochemical properties, making it ideal for electronic data processing. This terminology is not widely used in routine blood banking but serves as a standardized reference for research and complex cases.
- Antigen Numbers:
- D: Rh1 (uppercase “Rh” followed by number 1)
- C: Rh2
- E: Rh3
- c: Rh4 (lowercase “Rh” followed by number 4)
- e: Rh5
- Absence of Antigen: A hyphen (-) is placed before the corresponding number to indicate the absence of that specific antigen.
For example:
- Rh:1,2,3 – This represents a person who is Rh positive and has the D, C, and E antigens (D is Rh1, C is Rh2, and E is Rh3).
- Rh:-1,2,3 – This represents a person who is Rh negative (lacks D antigen, indicated by the hyphen before 1) but has the C, E, and e antigens (C is Rh2, E is Rh3, and e is Rh5, though typically e wouldn’t be specified as it’s usually present on Rh-negative individuals).
Common Rh Blood Group Haplotypes
Whites
The most frequent Rh haplotypes in whites are:
- R1 (CDe): This haplotype carries the C, D, and E antigens and is the most common in Caucasian populations, accounting for roughly 40-45% of all Rh haplotypes.
- r (ce): This haplotype lacks all major Rh antigens (C, D, and E) and is present in a smaller percentage, around 37% of Caucasian Rh haplotypes.
Blacks
The most frequent Rh haplotypes in blacks differ significantly:
- R0 (cDe): This is the most common haplotype in African American populations, accounting for roughly 40-45% of all Rh haplotypes.
- r (ce): This haplotype lacks all major Rh antigens (C, D, and E) and is present in a smaller percentage, around 36% of African American Rh haplotypes.
Anti-Rh Antibody Production
Rh-negative individuals lack the D antigen on their red blood cells (RBCs). This sets the stage for a potential immune response if they are exposed to Rh-positive blood.
Rh-Negative blood type and Anti-Rh Antibody Development
When Rh-negative individuals encounter Rh-positive blood (through transfusion or pregnancy with an Rh-positive fetus), their immune system recognizes the D antigen as foreign. This triggers the immune system to produce antibodies specifically targeting the D antigen, called anti-D antibodies. However, the first exposure usually doesn’t lead to a significant antibody response. It takes time for the immune system to develop these antibodies.
Immunoglobulin Class of Anti-Rh Antibodies
The primary class of anti-D antibodies produced is IgG (Immunoglobulin G). These are particularly concerning because they can cross the placenta in pregnant Rh-negative women carrying an Rh-positive fetus.
Secondary exposure to Rh-positive blood triggers a rapid and robust immune response in previously sensitized individuals. This can lead to significant antibody production, including:
- IgG Anti-D: The main culprit for causing hemolytic disease of the newborn (HDN) in Rh-positive babies born to sensitized Rh-negative mothers. IgG antibodies can cross the placenta and attack the baby’s Rh-positive RBCs, leading to anemia, jaundice, and potentially life-threatening complications.
- IgM (Immunoglobulin M) Anti-D: These antibodies are usually produced in smaller amounts but can also contribute to HDN, particularly in severe cases.
- Other Anti-Rh Antibodies: Besides anti-D, Rh-negative individuals can also develop antibodies against other Rh antigens (C, E, c, e) under certain circumstances. These can also cause complications in transfusions or pregnancies.
Techniques in Rh Blood Typing
Rh blood typing is crucial for ensuring safe blood transfusions and managing Rh-related complications in pregnancy.
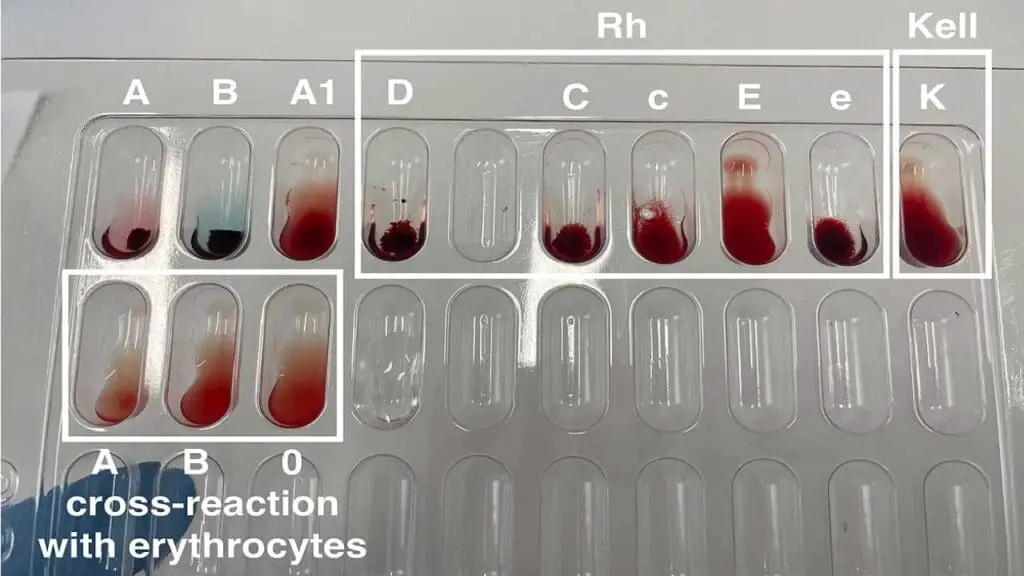
Agglutination Tests
- Principle: These tests rely on the ability of antibodies (anti-Rh) to bind to specific antigens (Rh antigens) on red blood cells, causing them to clump together (agglutinate).
- Two Main Techniques:
- Tube agglutination test (TT): This is a common method where red blood cells are mixed with anti-Rh sera in test tubes. After incubation, agglutination (clumping) is observed visually.
- Solid-phase assays: These assays utilize various platforms like microplates or beads coated with anti-Rh antibodies. Red blood cells are added, and agglutination is detected visually or electronically.
Additional Techniques
- Automated systems: Automated blood typing systems are gaining popularity, offering speed and reduced human error. They utilize similar principles of agglutination but with automated detection and interpretation.
- Indirect antiglobulin test (IAT): Indirect Antiglobulin Test (IAT) plays a crucial role in detecting weak D variants, a situation where standard Rh typing techniques might miss the presence of the D antigen. This test involves incubating the patient’s red blood cells with anti-D antibodies. However, unlike a regular agglutination test, an antiglobulin reagent is added afterwards. This “linking” reagent binds to any antibodies (including weak anti-D) that have attached to the red blood cells, causing visible agglutination and confirming the presence of a weak D antigen. This additional step enhances the sensitivity of the test, ensuring accurate Rh typing and preventing potential complications in blood transfusions and Rh-related pregnancies.
Clinical Significance of Rh Blood Group System
The Rh blood group system plays a vital role in blood transfusion compatibility and pregnancy outcomes.
Hemolytic Transfusion Reactions (Rh Incompatibility)
When an Rh-negative individual receives Rh-positive blood, their immune system recognizes the D antigen (or other Rh antigens) as foreign. This triggers the production of anti-Rh antibodies, which can attack and destroy the transfused Rh-positive red blood cells. This destruction leads to a hemolytic transfusion reaction, a potentially life-threatening condition with symptoms like fever, chills, hemoglobin in the urine (dark urine), and jaundice (yellowing of skin and eyes).
Rh Disease in Pregnancy (Erythroblastosis Fetalis)
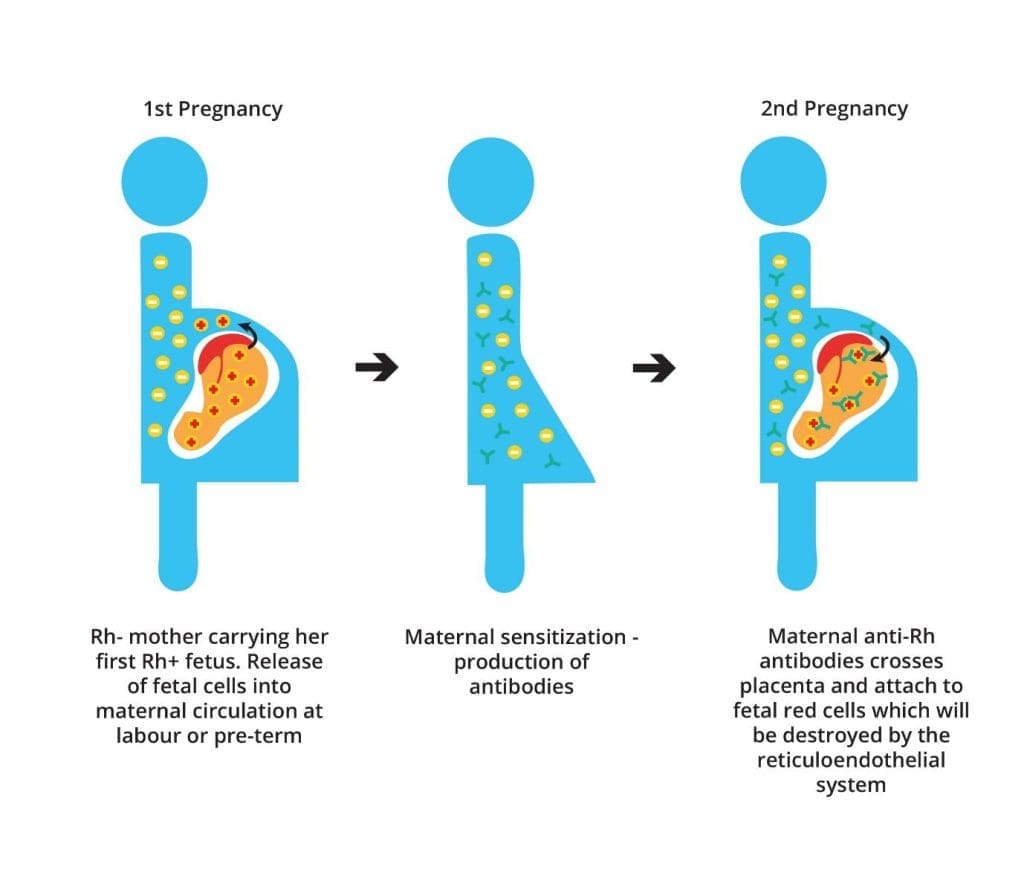
If an Rh-negative mother carries an Rh-positive fetus, fetal red blood cells with the D antigen can enter the mother’s circulation. During a first pregnancy, this usually doesn’t cause problems. However, the mother’s immune system can become sensitized to the D antigen. In subsequent pregnancies with Rh-positive fetuses, preformed anti-D antibodies can cross the placenta and attack the baby’s red blood cells. This leads to Rh disease in pregnancy (also called erythroblastosis fetalis or hemolytic fetal disease of the newborn), causing anemia, jaundice, and potentially life-threatening complications for the baby.
Management Strategies in Rh-Negative Pregnancies
To prevent Rh sensitization and protect the baby:
- Rh typing and antibody screening: Both mother and baby’s Rh status and presence of anti-Rh antibodies are determined.
- Rh immunoglobulin (RhIg) prophylaxis: Rh-negative mothers who are not sensitized receive RhIg injections during pregnancy and after delivery. RhIg binds to any fetal Rh-positive red blood cells that enter the mother’s circulation, preventing sensitization.
- Fetal monitoring: Close monitoring of the baby’s condition with tests like amniocentesis can be used to assess the severity of hemolytic disease and determine appropriate interventions if needed.
- Intrauterine transfusions: In severe cases, blood transfusions can be given to the fetus before birth to replace affected red blood cells.
Frequently Asked Questions (FAQs)
Is Rh and O the same blood type?
No, Rh and O are not the same blood type.
- O is a blood type within the ABO blood group system, determined by the presence or absence of A and B antigens on red blood cells.
- Rh is a separate blood group system, determined by the presence or absence of the D antigen on red blood cells.
Therefore, a person can have blood type O-positive, O-negative, A-positive, A-negative, B-positive, B-negative, AB-positive, or AB-negative. The “O” refers to the ABO system, and the positive or negative refers to the Rh system.
Why is Rh blood so rare?
Rh-negative blood is relatively rare because the D antigen is dominant. Most people inherit the D antigen, making them Rh-positive. The Rh-negative phenotype occurs only when both parents pass down the recessive gene for the lack of the D antigen. This genetic inheritance pattern results in a lower frequency of Rh-negative individuals compared to Rh-positive individuals.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Rosenkrans D, Zubair M, Doyal A. Rh Blood Group System. [Updated 2023 Aug 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK594252/
- Westhoff CM. The Rh blood group system in review: a new face for the next decade. Transfusion. 2004 Nov;44(11):1663-73. doi: 10.1111/j.0041-1132.2004.04237.x. Erratum in: Transfusion. 2005 Jan;45(1):125. PMID: 15504174.
- Moise KJ. Management of rhesus alloimmunization in pregnancy. Obstetrics & Gynecology. 2002; 100(3):600-11.