TL;DR
Thrombotic thrombocytopenic purpura (TTP) is a type of thrombocytopenia due to deficiency of ADAMTS13 metalloprotease which leads to formation of platelet thrombi in microvessels.
- Signs and symptoms (pentad) ▾:
- Thrombocytopenia
- Microangiopathic hemolysis
- Neurologic dysfunction
- Renal impairment
- Fever
- Causes ▾:
- Inherited mutations
- Acquired: inhibitory IgG autoantibodies stimulated by infection, autoimmune/connective tissue disease, drugs, stem cell transplantation or cardiac surgery
- Pathophysiology ▾: TTP is primarily due to a deficiency or dysfunction of ADAMTS13. This deficiency leads to the formation of abnormally large VWF multimers, which can bind platelets excessively, triggering platelet aggregation and thrombus formation. These microthrombi can occlude small blood vessels, leading to tissue damage.
- Laboratory investigations ▾:
- Full blood count: Thrombocytopenia
- Peripheral blood smear: Schistocytosis
- Bone marrow characteristics: Markedly hypercellular with erythroid hyperplasia
- ↑↑ serum LDH
- Coagulation tests normal
- Specific assay: ADAMTS-13 ↓↓ or absent
- Treatment and management ▾:
- Plasma exchange using FFP or cryosupernatant
- Rituximab
*Click ▾ for more information
Introduction
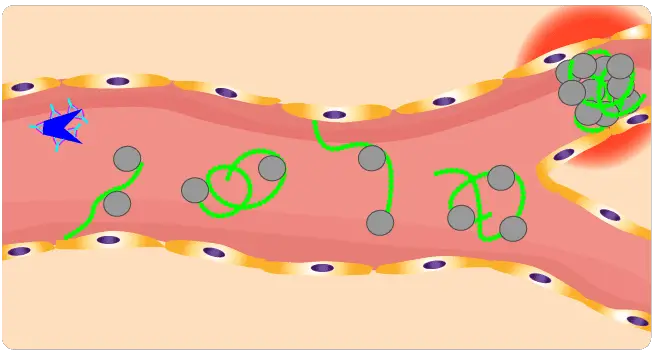
Thrombotic thrombocytopenic purpura (TTP) is a rare and life-threatening blood disorder. It is characterized by the formation of small blood clots (microthrombi) throughout the body. These microthrombi can block small blood vessels and damage organs, such as the brain, kidneys, and heart.
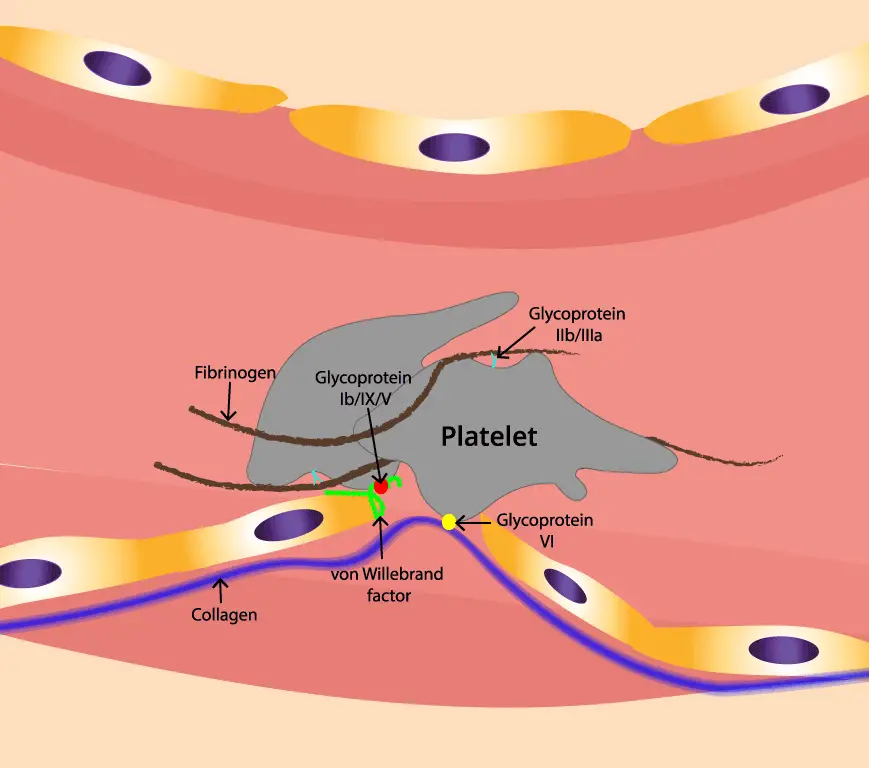
Thrombotic thrombocytopenic purpura (TTP) is caused by a deficiency of a protein called ADAMTS13. ADAMTS13 is responsible for cleaving a large protein called von Willebrand factor (VWF).
VWF acts as a bridge between platelets and the walls of blood vessels at the site of an injury by binding to collagen and capturing platelets. VWF also stabilizes factor VIII, another essential clotting protein. When ADAMTS13 is deficient, VWF forms large multimers that can clump together to form microthrombi.
There are two main types of thrombotic thrombocytopenic purpura (TTP):
- Acquired: This is the most common type of thrombotic thrombocytopenic purpura (TTP) and is caused by an autoimmune disorder. In autoimmune disorders, the body’s immune system attacks its own tissues. In acquired thrombotic thrombocytopenic purpura (TTP), the immune system attacks ADAMTS13.
- Congenital: This is caused by a genetic mutation that affects the ADAMTS13 gene and is rare.
Pathophysiology of Thrombotic Thrombocytopenic Purpura (TTP) & ADAMTS13
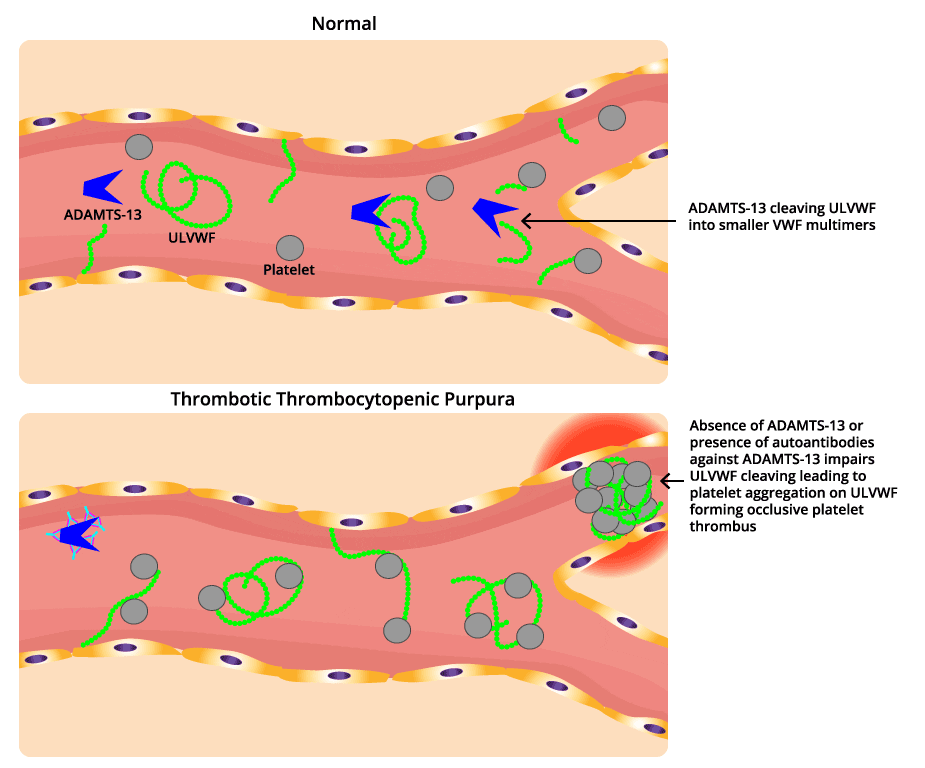
ADAMTS-13, an enzyme responsible for cleaving large von Willebrand factor (VWF) multimers, plays a pivotal role in maintaining the delicate balance of blood vessel integrity. In thrombotic thrombocytopenic purpura (TTP), the absence of ADAMTS-13 leads to an accumulation of these large VWF multimers, creating a sticky environment that promotes platelet aggregation.
This disorder occurs in familial or acquired forms.
Inherited TTP Pathophysiology
The inherited pathophysiology of Thrombotic Thrombocytopenic Purpura (TTP) originates from a genetic mutation in the ADAMTS13 gene. This mutation results in the absence of the ADAMTS13 metalloprotease, an enzyme vital for cleaving large von Willebrand factor (VWF) multimers.
In the absence of functional ADAMTS13, these ultra-large VWF multimers accumulate. This accumulation creates an environment where platelets excessively adhere to these VWF strings via their GP1bɑ receptors, leading to increased platelet aggregation. This abnormal aggregation can form large, occlusive platelet thrombi within microvessels, which may then embolize, leading to organ ischemia and the characteristic symptoms of TTP.
Acquired TTP Pathophysiology
The acquired pathophysiology of Thrombotic Thrombocytopenic Purpura (TTP) is primarily an autoimmune disorder, making it the most common form of TTP. In this condition, the body’s immune system mistakenly produces an inhibitory IgG autoantibody that attacks the ADAMTS13 enzyme.
When ADAMTS13 is deficient or dysfunctional due to this autoimmune attack, abnormally large VWF multimers accumulate in the bloodstream.
These accumulated large VWF multimers excessively bind to platelets, triggering their aggregation and leading to the formation of microthrombi (small blood clots) within the small blood vessels throughout the body. These microthrombi can then occlude these vessels, causing tissue damage and the characteristic symptoms of TTP, including thrombocytopenia, hemolytic anemia, fever, neurologic changes, and renal dysfunction.
The development of this autoantibody can be stimulated by various factors such as infection, autoimmune diseases, certain drugs, stem cell transplantation, or cardiac surgery.
Clinical Signs and Symptoms of TTP
Thrombotic Thrombocytopenic Purpura (TTP) is characterized by a range of symptoms, often summarized as a classic pentad, though clinical manifestations can vary. The core features include:
- Thrombocytopenia: This refers to a low platelet count, which occurs because platelets are consumed in the formation of numerous small blood clots (microthrombi) throughout the body’s microvessels.
- Microangiopathic Hemolytic Anemia (MAHA): This type of anemia develops as red blood cells are fragmented when they attempt to pass through the narrowed, clot-filled microvessels.
- Neurologic Dysfunction: Microthrombi affecting the brain can lead to a variety of neurological symptoms. These can range from mild manifestations like headaches and confusion to more severe issues such as seizures or specific focal neurologic deficits.
- Renal Impairment: When these small clots impact the kidneys, it can result in impaired kidney function, potentially leading to kidney failure.
- Fever: This is also a commonly observed symptom in individuals with TTP.
Beyond the classic pentad, other clinical manifestations that may be observed include generalized fatigue and pallor (unusual paleness). Histologically, a kidney biopsy might reveal “onion skinning,” which refers to concentric layers of fibrinoid material in blood vessel walls, a significant finding indicating vascular occlusive disease and supporting a TTP diagnosis.
Laboratory Investigations of TTP
Complete Blood Count (CBC) with Platelet Count
- Severe Thrombocytopenia: This is a hallmark of TTP, with platelet counts typically very low, often below 30,000/μL. This reflects the consumption of platelets in the formation of microthrombi.
- Anemia: The CBC will also show anemia, which is microangiopathic hemolytic anemia (MAHA).
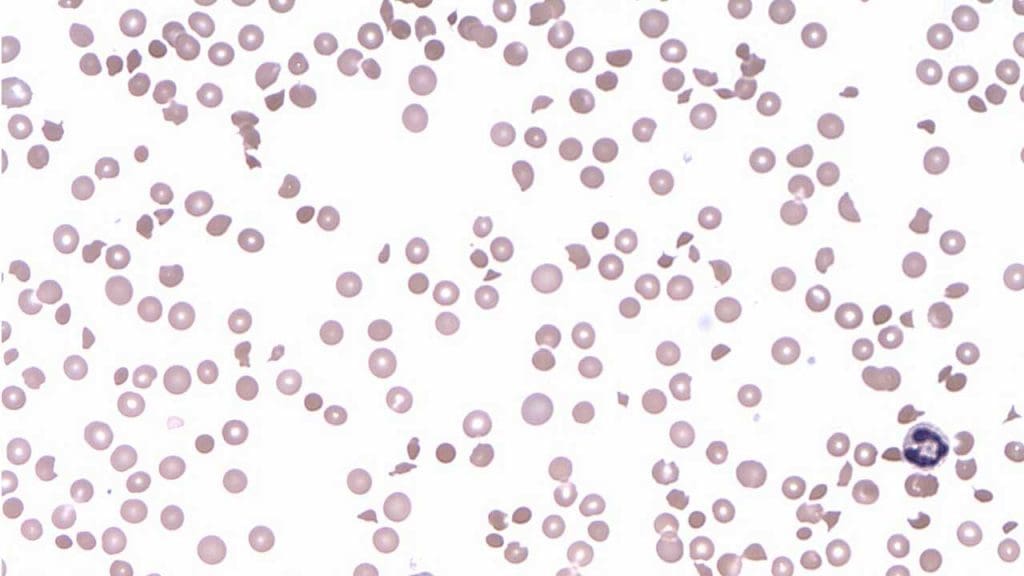
Peripheral Blood Smear
- Schistocytes: The presence of abnormal red blood cell morphology, schistocytes (fragmented red blood cells), on the peripheral blood smear is a critical diagnostic finding. These fragments are formed as red blood cells are sheared when passing through small blood vessels partially occluded by platelet thrombi.
- Polychromatophilia: This may also be observed, indicating an increased production of immature red blood cells by the bone marrow in an attempt to compensate for the accelerated red blood cell destruction.
Markers of Hemolysis
- Elevated Lactate Dehydrogenase (LDH): LDH is an enzyme released from damaged red blood cells and tissues. Elevated levels indicate significant hemolysis and tissue injury due to microthrombi.
- Decreased Haptoglobin: Haptoglobin is a protein that binds free hemoglobin released from destroyed red blood cells. In hemolytic anemia, haptoglobin levels are typically low or undetectable as it gets consumed.
- Elevated Indirect Bilirubin: This is a breakdown product of hemoglobin, and its elevation indicates increased red blood cell destruction.
- Elevated Reticulocyte Count: The bone marrow attempts to compensate for red blood cell destruction by increasing the production of new, immature red blood cells (reticulocytes), leading to an elevated count.
- Negative Direct Coombs Test: This test is crucial to differentiate TTP from immune-mediated hemolytic anemias. A negative result in the presence of MAHA supports a diagnosis of TTP, as the hemolysis in TTP is mechanical (due to shear stress from thrombi), not antibody-mediated.
ADAMTS13 Activity and Inhibitor Assays
- Severely Reduced ADAMTS13 Activity (<10%): This is the definitive diagnostic test for TTP. A severe deficiency in the ADAMTS13 enzyme confirms the diagnosis in patients with clinical evidence of MAHA and thrombocytopenia.
- ADAMTS13 Inhibitor (Antibody) Assay: In acquired TTP (the most common form), an inhibitory autoantibody against ADAMTS13 is present. Detecting this antibody helps differentiate acquired TTP from the inherited form (Upshaw-Schulman syndrome), where the deficiency is due to genetic mutations and no autoantibody is present.
Coagulation Studies (PT, aPTT, Fibrinogen)
Normal Coagulation Profile: Unlike Disseminated Intravascular Coagulation (DIC), which can have similar symptoms, TTP typically presents with normal prothrombin time (PT), activated partial thromboplastin time (aPTT), and fibrinogen levels. This helps in differentiating TTP from other thrombotic microangiopathies.
Renal Function Tests
Creatinine: While severe renal impairment is less common in TTP compared to some other thrombotic microangiopathies, elevated creatinine levels can indicate kidney involvement due to microthrombi.
PLASMIC Score
This is a clinical prediction score that utilizes readily available laboratory and clinical parameters at presentation to estimate the likelihood of severe ADAMTS13 deficiency, helping clinicians make a presumptive diagnosis rapidly while awaiting definitive ADAMTS13 assay results.
Prompt initiation of treatment based on clinical suspicion and initial laboratory findings is critical due to the high mortality rate of untreated TTP, even before confirmatory ADAMTS13 activity results are available.
Blood Vessels with Onion Skinning in TTP Kidney Biopsy
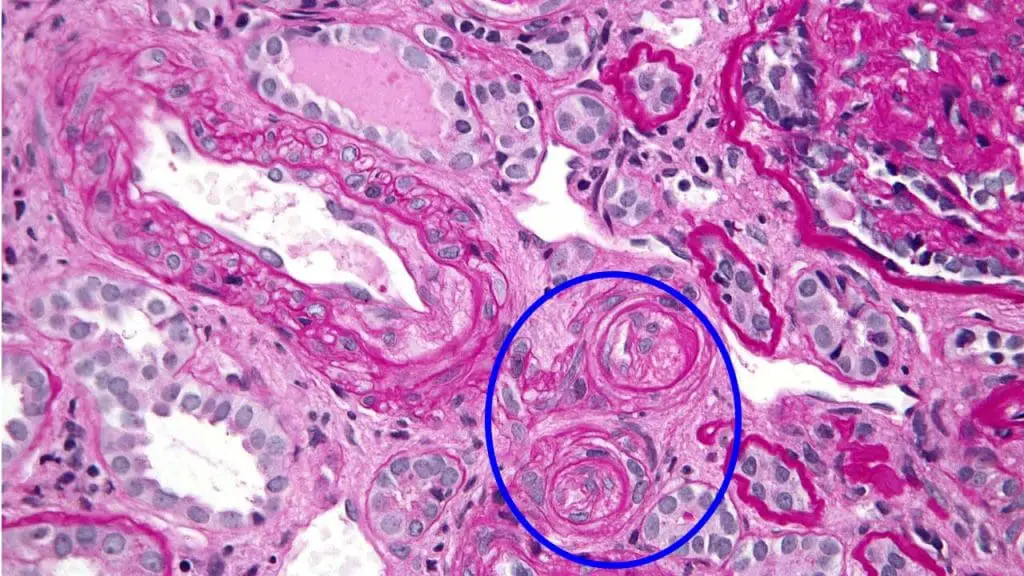
Onion skinning refers to a specific histological finding in kidney biopsies. It describes the appearance of blood vessel walls with concentric layers of fibrinoid material. This layering resembles the skin of an onion, hence the name.
In the context of thrombotic thrombocytopenic purpura (TTP), onion skinning is a significant finding and indicates vascular occlusive disease.
The mechanism behind onion skinning in thrombotic thrombocytopenic purpura (TTP) involves the deposition of platelet-fibrin thrombi within small blood vessels, particularly the arterioles. These thrombi consist of platelets, fibrin, and other blood components. As the thrombi accumulate, they cause the vessel walls to become thickened and layered, leading to the characteristic onion-skinning appearance.
Differential Diagnosis of TTP
The differential diagnosis of Thrombotic Thrombocytopenic Purpura (TTP) is crucial because TTP shares clinical and laboratory features with several other life-threatening conditions, particularly other Thrombotic Microangiopathies (TMAs). Accurate differentiation is critical for appropriate and timely treatment, as therapies vary significantly.
| Condition | Key Clinical Features | Distinguishing Laboratory/Pathophysiological Features |
| Thrombotic Thrombocytopenic Purpura (TTP) | Classic pentad: Thrombocytopenia, MAHA, Neurologic dysfunction, Renal impairment (often mild), Fever. | Severely deficient ADAMTS13 activity (<10%). Acquired form: presence of ADAMTS13 autoantibody. Normal PT/aPTT, normal fibrinogen. |
| Hemolytic Uremic Syndrome (HUS) | ||
| Typical HUS (STEC-HUS) | Preceded by diarrheal illness (often bloody). Prominent acute kidney injury, thrombocytopenia, MAHA. | Normal ADAMTS13 activity (>10%). Severe renal involvement is dominant. Shiga toxin detection or culture. |
| Atypical HUS (aHUS) | Thrombocytopenia, MAHA, prominent acute kidney injury. Can recur. | Normal ADAMTS13 activity (>10%). Evidence of complement pathway dysregulation (e.g., genetic mutations in complement proteins, low C3). |
| Disseminated Intravascular Coagulation (DIC) | Bleeding and thrombosis. Often triggered by sepsis, malignancy, trauma, obstetric emergencies. | Abnormal coagulation parameters: Prolonged PT/aPTT, low fibrinogen, elevated D-dimer. ADAMTS13 activity usually normal. |
| HELLP Syndrome | Occurs in pregnancy/postpartum. Hemolysis, elevated liver enzymes, low platelets. Often with hypertension, proteinuria. | Normal or mildly reduced ADAMTS13 activity (rarely <10%). Elevated liver enzymes (ALT/AST). Occurs in context of preeclampsia/eclampsia. |
| Drug-Induced Thrombotic Microangiopathy (DITMA) | Occurs after exposure to specific drugs (e.g., quinine, ticlopidine, clopidogrel, gemcitabine, cyclosporine, tacrolimus, bevacizumab). | Normal ADAMTS13 activity (>10%). Resolution upon drug discontinuation. Drug-dependent antibodies may be present. |
| Other Secondary TMAs | Clinical features depend on underlying condition (e.g., cancer, autoimmune disease like SLE, post-HSCT, malignant hypertension, HIV). | Normal or moderately reduced ADAMTS13 activity (usually >10%). Evidence of underlying systemic disease. Treatment directed at the underlying cause. |
Treatment and Management
The treatment and management of Thrombotic Thrombocytopenic Purpura (TTP) are critical due to its life-threatening nature, focusing on two main objectives: increasing functional ADAMTS13 enzyme levels and preventing the formation of harmful microthrombi.
- Plasma Exchange (PEX) / Plasmapheresis: PEX is the primary and most vital treatment for acute TTP, especially in acquired forms. It should be initiated urgently upon strong clinical suspicion, even before definitive ADAMTS13 assay results are available. During PEX, the patient’s plasma, which contains the inhibitory autoantibodies and ultra-large von Willebrand factor (VWF) multimers, is removed and replaced with fresh frozen plasma (FFP) from donors. This process achieves two crucial goals:
- Provides functional ADAMTS13: FFP supplies the deficient ADAMTS13 enzyme.
- Removes pathogenic autoantibodies: It clears the autoantibodies that are inhibiting the patient’s own ADAMTS13.
- Corticosteroids (e.g., prednisone, methylprednisolone): Often administered concurrently with plasma exchange, particularly in acquired (autoimmune) TTP. Corticosteroids are immunosuppressants that help to reduce the production of autoantibodies against ADAMTS13 and dampen the inflammatory response.
- Immunosuppressive Agents (e.g., Rituximab): This is a monoclonal antibody that targets CD20-positive B cells. Since B cells are responsible for producing the autoantibodies against ADAMTS13 in acquired TTP, rituximab helps to deplete these B cells, thereby reducing autoantibody production. It is often used for patients with acquired TTP who are at high risk of relapse, have refractory disease (not responding well to PEX and corticosteroids), or for maintenance therapy to prevent future episodes.
- Caplacizumab: Caplacizumab is a novel humanized single variable domain antibody fragment that specifically targets the A1 domain of von Willebrand factor. By blocking the interaction between ultra-large VWF multimers and platelets, caplacizumab directly inhibits platelet aggregation and the formation of microthrombi, regardless of ADAMTS13 activity. It has been shown to achieve faster platelet count normalization, reduce the need for repeat plasma exchange, and significantly lower the risk of TTP-related death and recurrence. It is used in conjunction with PEX and immunosuppression.
- Long-Term Management and Monitoring: TTP often requires long-term management due to the risk of relapse, especially with the acquired form.
- Regular Monitoring: Patients need ongoing monitoring of ADAMTS13 activity levels and antibody titers, even during remission.
- Relapse Prevention: Some patients may require long-term immunosuppressive therapy or intermittent treatment with agents like rituximab to prevent future flares.
- Patient Education: Educating patients about their condition, potential triggers, and the importance of adhering to their treatment plan and follow-up appointments is crucial.
The goal of these comprehensive treatments is to rapidly control acute episodes, prevent organ damage, minimize the risk of relapse, and improve the patient’s overall quality of life.
Frequently Asked Questions (FAQs)
Can you live a normal life with thrombotic thrombocytopenic purpura (TTP)?
Living with TTP can be challenging, but it is possible to lead a relatively normal life with proper management. The key is to work closely with a healthcare team that specializes in TTP. Treatment often involves plasma exchange therapy to remove harmful antibodies and replace deficient ADAMTS13. Additionally, corticosteroids may be used to suppress the immune system.
While there may be limitations, especially during acute flares, many individuals with TTP can maintain a good quality of life with consistent medical care and lifestyle adjustments.
What are the triggers of thrombotic thrombocytopenic purpura (TTP)?
Triggers of thrombotic thrombocytopenic purpura (TTP) can vary, but some common factors include:
- Infections: Viral or bacterial infections can sometimes trigger thrombotic thrombocytopenic purpura (TTP).
- Pregnancy: Thrombotic thrombocytopenic purpura (TTP) can occur during or after pregnancy.
- Medications: Certain medications, such as immunosuppressants, antiplatelet drugs, or antibiotics, may be associated with thrombotic thrombocytopenic purpura (TTP).
- Underlying medical conditions: Autoimmune disorders or other medical conditions can increase the risk of thrombotic thrombocytopenic purpura (TTP).
- Surgery: Major surgeries or trauma can sometimes lead to thrombotic thrombocytopenic purpura (TTP).
It’s important to note that not everyone with these factors will develop thrombotic thrombocytopenic purpura (TTP), and sometimes the cause remains unknown.
Can thrombotic thrombocytopenic purpura (TTP) be cured?
TTP is not typically curable. While treatment can effectively manage the condition and prevent life-threatening complications, it often requires lifelong monitoring and management. The goal of treatment is to control symptoms, prevent future flares, and improve overall quality of life.
What is the difference between TTP and immune thrombocytopenia (ITP)?
TTP (Thrombotic Thrombocytopenic Purpura) and ITP (Immune Thrombocytopenia) are both blood disorders that affect platelets, but they have different underlying causes and mechanisms.
ITP (Immune Thrombocytopenia) is an autoimmune disorder where the body’s immune system mistakenly attacks and destroys platelets. This results in a low platelet count (thrombocytopenia), leading to easy bruising and bleeding.
TTP (Thrombotic Thrombocytopenic Purpura) is a disorder caused by a deficiency or dysfunction of an enzyme called ADAMTS13. This enzyme is responsible for breaking down large von Willebrand factor (VWF) molecules. When ADAMTS13 is not working properly, these large VWF molecules can cause blood clots to form in small blood vessels throughout the body. This can lead to a variety of symptoms, including low platelet count, anemia, fever, neurologic problems, and kidney failure.
While both Thrombotic Thrombocytopenic Purpura (TTP) and Immune Thrombocytopenia (ITP) are blood disorders that cause a low platelet count (thrombocytopenia) and can lead to symptoms like easy bruising and bleeding, they have fundamentally different causes, clinical features, and treatments. The key difference lies in their underlying mechanism: TTP is a disorder of widespread clotting in small blood vessels, while ITP is an autoimmune disorder of platelet destruction.
Comparison
| Feature | Thrombotic Thrombocytopenic Purpura (TTP) | Immune Thrombocytopenia (ITP) |
| Pathophysiology | Caused by a severe deficiency of the ADAMTS13 enzyme, which leads to the accumulation of large von Willebrand factor (vWF) multimers. These multimers spontaneously bind to platelets, causing widespread microclots to form in small blood vessels. | An autoimmune disorder where the immune system, specifically B and T cells, produces antibodies that mistakenly target and destroy platelets in the spleen and other organs. |
| Mechanism of Thrombocytopenia | Platelets are consumed (used up) as they form numerous microclots throughout the body’s small vessels. | Platelets are destroyed by the immune system, leading to a peripheral decrease in their count. |
| Associated Clinical Features | Part of a “pentad” of symptoms, including: thrombocytopenia, fever, neurological symptoms (e.g., confusion, seizures, headache), kidney dysfunction, and microangiopathic hemolytic anemia (MAHA), where red blood cells are sheared and destroyed as they pass through the microclots. | Isolated thrombocytopenia. While bleeding can be severe, it is not typically accompanied by the systemic organ damage, fever, or MAHA seen in TTP. |
| Lab Findings | CBC: Very low platelet count (<20,000/µL). Signs of hemolysis: Elevated LDH, elevated indirect bilirubin, decreased haptoglobin, and the presence of schistocytes (fragmented red blood cells) on a peripheral blood smear. Coagulation studies (PT/PTT): Normal. Renal profile: Elevated creatinine (indicating kidney injury). | CBC: Low platelet count (often <30,000/µL). Hemoglobin and other blood cell counts are generally normal. Coagulation studies (PT/PTT): Normal. PBS: The peripheral blood smear shows a low number of platelets but is otherwise normal, without schistocytes. |
| Urgency of Treatment | A medical emergency. Without immediate treatment, TTP has a high mortality rate. | Can be monitored in many cases, especially in children, and may not require immediate treatment unless there is significant bleeding or the platelet count is extremely low. |
| First-line Treatment | Plasma exchange (PLEX), which removes the circulating antibodies against ADAMTS13 and replaces the deficient enzyme. It is often combined with immunosuppressive drugs like corticosteroids. | Corticosteroids (e.g., prednisone) to suppress the immune system’s attack on platelets. Intravenous immunoglobulin (IVIG) may also be used for a more rapid increase in platelet count. |
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Kovala M, Seppälä M, Kaartinen K, Meri S, Honkanen E, Räisänen-Sokolowski A. Vascular Occlusion in Kidney Biopsy Is Characteristic of Clinically Manifesting Thrombotic Microangiopathy. Journal of Clinical Medicine. 2022; 11(11):3124. https://doi.org/10.3390/jcm11113124.
- UpToDate – Diagnosis of Immune TTP.
- Nuñez Zuno JA, Khaddour K. Thrombotic Thrombocytopenic Purpura Evaluation and Management. [Updated 2023 Sep 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-.
- Inés Gómez-Seguí, Cristina Pascual Izquierdo, María Eva Mingot Castellano & Javier de la Rubia Comos (2023) An update on the pathogenesis and diagnosis of thrombotic thrombocytopenic purpura, Expert Review of Hematology, 16:1, 17-32, DOI: 10.1080/17474086.2023.2159803
- Rizzo C, Rizzo S, Scirè E, Di Bona D, Ingrassia C, Franco G, Bono R, Quintini G, Caruso C. Thrombotic thrombocytopenic purpura: a review of the literature in the light of our experience with plasma exchange. Blood Transfus. 2012 Oct;10(4):521-32. doi: 10.2450/2012.0122-11. Epub 2012 Jun 27. PMID: 22790258; PMCID: PMC3496241.
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Stanley M, Killeen RB, Michalski JM. Thrombotic Thrombocytopenic Purpura. [Updated 2023 Apr 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430721/
- Sukumar, S., Lämmle, B., & Cataland, S. R. (2021). Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management. Journal of Clinical Medicine, 10(3), 536. https://doi.org/10.3390/jcm10030536