TL;DR
| Blood Product | Storage | Transport | Expiry | Indications |
| Whole blood | In a blood bank refrigerator at 2℃ – 6℃ | Can be transported for the next 24h if maintained at 1℃ – 10℃ during transportation | 35 days in a closed system, 24 hr in an open system | Acute blood loss Exchange transfusion Massive transfusion |
| Packed red cells | In a blood bank refrigerator at 2℃ – 6℃ | Can be transported for the next 24h if maintained at 1℃ – 10℃ during transportation | 35 days in a closed system, 24 hr in an open system | Chronic, symptomatic anemia Acute blood loss |
| Fresh frozen plasma | In a plasma freezer at below -30℃ | Transported in frozen state | 1 year | Multiple factor deficiencies Severed liver disease Warfarin reversal |
| Platelet concentrate | In a platelet incubator with agitator at 20℃ – 24℃ with continuous gentle agitation | 20℃ – 24℃ | 5 days in a closed system, 4 hr in an open system | Bleeding due to low platelet count or impaired function |
| Cryoprecipitate | In a freezer at below -30℃ | Below -30℃ | 1 year | Fibrinogen deficiency Dysfibrinogenemia Trauma DIC |
What happens to donated blood after blood donation?
Blood is a complex fluid that contains a variety of components, each with its own unique function. After blood collection of donated blood, blood can be separated into different blood product components for storage and used for transfusion to treat a variety of medical conditions.
Preparation Process of Blood Product
Donated blood products are obtained through two main methods: the traditional Whole Blood Donation followed by blood component separation, and the specialized process of Apheresis. Understanding these methods highlights the complex laboratory work involved in transfusion medicine.
Whole Blood Donation and Blood Component Separation
Whole blood donation is the most common method of donation. Approximately 450–500 mL of blood is collected from the donor into a specialized bag containing an anticoagulant and preservative solution.
The Separation Process
- Collection: A standard unit of Whole Blood is collected.
- Centrifugation: The whole blood unit is placed in a high-speed centrifuge. The difference in density of the cellular components causes the blood to separate into three distinct layers:
- Plasma (Top Layer): The least dense, straw-colored liquid component. This is often processed immediately and rapidly frozen to create Fresh Frozen Plasma (FFP).
- Buffy Coat (Middle Layer): A thin, whitish layer containing the majority of the White Blood Cells (Leukocytes) and Platelets.
- Packed Red Blood Cells (Bottom Layer): The most dense component, containing the oxygen-carrying red cells. A preservative solution is added to increase its shelf life.
- Extraction: Specialized equipment then physically separates these layers into their final bags using a sterile, closed system. The platelets can be further processed from multiple buffy coats to create a therapeutic dose of pooled platelet concentrate.
Apheresis (Blood Component Specific Collection)
Apheresis (from the Greek word meaning “to take away”) is a specialized collection process where a specific blood component is selectively removed, and the remaining blood components are returned to the donor. This technique is more time-consuming but offers distinct advantages. The main clinical benefit of apheresis is reducing donor exposure for the recipient. When a patient receives platelets from a single apheresis donor, they are exposed to the antigens and infectious disease risks of only one person, rather than five or six people.
The Apheresis Process
- Blood Draw: Blood is drawn from the donor, typically from one arm.
- Component Separation: The blood is channeled into an automated cell separator machine. This machine uses either high-speed centrifugation (like a continuous flow centrifuge) or selective filtration to isolate the desired blood component (e.g., platelets, plasma, or sometimes red cells).
- Return: The remaining blood components (which the donor does not need to lose) are continuously returned to the donor, usually through the same needle or a needle in the opposite arm.
| Blood Component Collected via Apheresis | Key Benefit |
| Apheresis Platelets | A single donation yields a full therapeutic dose of platelets, equivalent to pooling platelets from 4–6 whole blood donations. This reduces the donor exposure for the patient. |
| Apheresis Plasma | Can be collected in larger volumes than from whole blood, and the process allows the donor to donate more frequently. |
| Double Red Cell Apheresis | Allows the collection of two units of Packed Red Blood Cells (PRBCs) from a single donor, which is highly efficient. |
What are the common blood components derived from donated blood and what are they used for?
Whole Blood
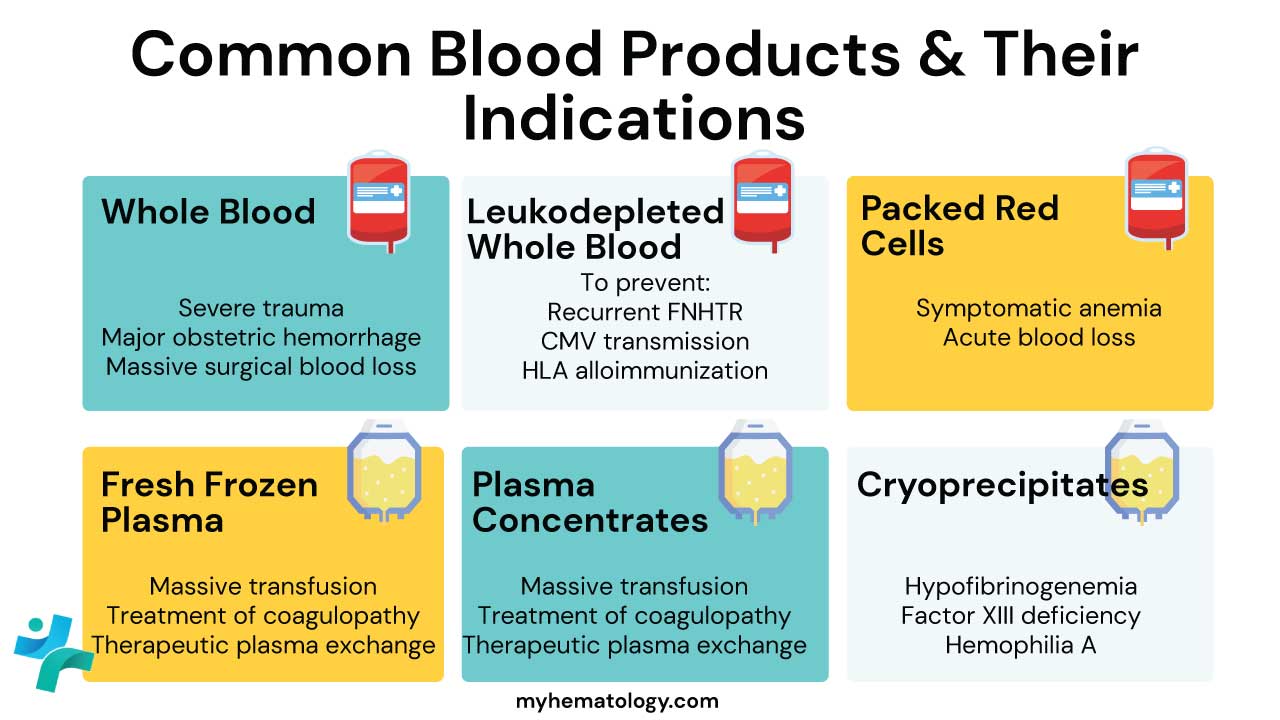
Whole blood (WB) is a specialized blood product that contains all the original components of blood and is primarily used for massive, life-threatening hemorrhage.
While it was the standard product historically, it is not the most common product today. Modern transfusion medicine favors component therapy (using Packed Red Blood Cells, Plasma, and Platelets separately) for most indications.
Whole blood has seen a resurgence in recent years, particularly in trauma and military settings, due to its unique benefits in hemorrhagic shock.
Storage and Transportation Conditions of Blood Product
Whole blood should be stored at 1-6°C and transported in a temperature-controlled environment. This blood product has a shelf life of 21 to 35 days depending on the anticoagulant or preservative used.
Indications for Use of Blood Product
Whole blood is reserved for situations of Massive Hemorrhage where the patient is losing all blood components (red cells, plasma, and platelets) rapidly and requires immediate, balanced resuscitation.
The primary indication is:
- Massive Transfusion Protocol (MTP) Activation in patients with life-threatening, uncontrolled bleeding due to:
- Severe Trauma (e.g., major vehicle accidents, penetrating injuries).
- Major Obstetric Hemorrhage.
- Massive Surgical Blood Loss.
Leukodepleted Whole Blood
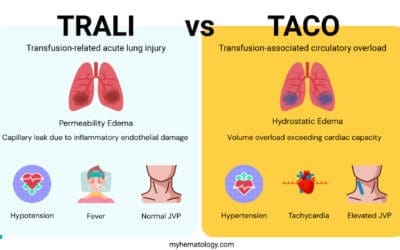
Whole blood can also be leucodepleted which must be performed soon after collection and prior to processing. A blood component is defined as leucocyte-depleted if there are less than 5 x 106/L of white cells present. Leukodepleted whole blood is whole blood that has had the white blood cells removed. This is done to reduce the risk of adverse reactions to blood transfusions, such as febrile non-hemolytic transfusion reactions (FNHTRs) and transfusion-related acute lung injury (TRALI). It is effective at preventing transmission of cytomegalovirus in countries where this has been reported.
Storage and Transportation Conditions of Blood Product
Leukodepleted whole blood product should be stored at 1-6°C and transported in a temperature-controlled environment. It has a shelf life of 21 – 35 days.
Indications for Use of Blood Product
The use of leukodepleted whole blood combines the indications for whole blood (massive hemorrhage) with the indications for leukodepletion (risk reduction).
Primary Indication (Whole Blood Use)
- Massive Transfusion Protocol (MTP) Activation: In the setting of massive, life-threatening hemorrhage (e.g., trauma, major surgery, battlefield injury) where the patient is losing all blood components simultaneously and requires immediate, balanced resuscitation.
Leukodepleted whole blood may also be used to treat patients who need a transfusion of whole blood, but who are at risk of transmitting infections through white blood cells. This may include patients with:
- HIV/AIDS
- Cytomegalovirus (CMV)
- Epstein-Barr virus (EBV)
- Human T-lymphotropic virus (HTLV)
Packed Red Blood Cells (PRBCs)
Packed red blood cells (PRBCs) are a concentrated preparation of red blood cells. This blood product is prepared by removing most of the plasma from whole blood. PRBCs are typically used to treat patients who have anemia. Packed red cells are the treatment of choice for most transfusions including chronic anemia.
Storage and Transportation Conditions of Blood Product
PRBCs blood product should be stored at 1-6°C and transported in a temperature-controlled environment. They have a shelf life of 21 – 42 days depending on additive solutions.
Indications for Use of Blood Product
- Anemia
- Chronic blood loss
- Surgery
PRBCs may also be used to treat patients with other conditions, such as:
- Heart disease
- Sickle cell disease
- Thalassemia
- Cancer
- Surgery
Benefits of PRBCs
PRBCs have a number of benefits over whole blood, including:
- PRBCs can be transported more easily than whole blood.
- PRBCs are less likely to cause adverse reactions than whole blood.
Fresh Frozen Plasma (FFP)
Fresh frozen plasma is a blood component which contains labile clotting factors and other constituents for transfusion or fractionation. It is rapidly frozen after separation to achieve complete freezing within 1 hour to a core temperature of -30°C. Its main use is for the replacement of coagulation factors or after massive transfusion, in liver disease and DIC, after cardiopulmonary bypass surgery, to reverse warfarin effect and in thrombotic thrombocytopenic purpura.
Storage and Transportation Conditions of Blood Product
FFP should be stored at -18°C or colder and transported in a temperature-controlled environment. It has a shelf life of 36 months if stored at or below -25°C and 3 months if stored at -20°C.
Indications for Use of Blood Product
FFP is typically used to treat patients with the following conditions:
- Bleeding disorders, such as hemophilia A, hemophilia B, and von Willebrand disease
- Liver disease
- Massive blood loss
- Warfarin reversal
- Thrombotic thrombocytopenic purpura (TTP)
- Hemolytic uremic syndrome (HUS)
Benefits of FFP
FFP has a number of benefits, including:
- It is a source of clotting factors, which can help to control bleeding.
- It contains proteins that can help to maintain blood volume and prevent shock.
- It can be used to treat a variety of bleeding disorders.
Platelet Concentrates
Platelet concentrates are a preparation of platelets. Platelet concentrates can be pooled from four donors of the same blood type to form 1 unit or from plateletpheresis. Platelet transfusion is used in patients who are thrombocytopenic or have platelet function disorders and who are actively bleeding as a therapeutic use or are at serious risk of bleeding as a prophylactic use.
Platelet transfusions should be avoided in autoimmune thrombocytopenic purpura unless there is serious hemorrhage. They are also contraindicated in heparin-induced thrombocytopenia, thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Platelets expressing HLA class I antigens and HLA-matched or crossmatch-compatible platelets are needed for patients with HLA antibodies.
Storage and Transportation Conditions of Blood Product
Platelets are to be stored at 20-24°C under constant agitation throughout storage and with a shelf life of 5 days.
Indications for Use of Blood Product
Platelet concentrates are typically used to treat patients with the following conditions:
- Low platelet count (thrombocytopenia)
- Bleeding disorders, such as idiopathic thrombocytopenic purpura (ITP) and thrombocytopenia associated with leukemia or chemotherapy
- Massive blood loss
- Surgery
- Liver disease
Benefits of Platelet Concentrates
Platelet concentrates have a number of benefits, including:
- They can help to prevent bleeding in patients with low platelet counts or who are at risk of bleeding.
- They can help to control bleeding in patients with bleeding disorders.
- They are generally well-tolerated by patients.
Cryoprecipitate
Cryoprecipitate is a preparation of clotting factors that is derived from FFP. Cryoprecipitate is typically used to treat patients who have bleeding disorders, such as hemophilia A or von Willebrand disease.
Cryoprecipitate is obtained by thawing FFP at 4°C and contains concentrated factor VIII and fibrinogen. After thawing, the component is re-centrifuged using a hard spin at the same temperature. The supernatant, cryo-poor plasma is then partially removed while the remaining cryo-poor plasma is used for resuspension. The resulting cryoprecipitate is then rapidly frozen.
Storage and Transportation Conditions of Blood Product
This blood product is stored at less than -30°C or if lyophilized at 4 – 6°C. It has a shelf life of 1 year.
Indications for Use of Blood Product
Cryoprecipitate is typically used to treat patients with the following conditions:
- Bleeding disorders, such as von Willebrand disease and hemophilia A
- Massive blood loss
- Thrombotic thrombocytopenic purpura (TTP)
- Hemolytic uremic syndrome (HUS)
- Fibrinogen deficiency
- Hypofibrinogenemia
- Factor XIII deficiency
- Massive transfusion
- Hepatic failure
Benefits of Cryoprecipitate
Cryoprecipitate has a number of benefits, including:
- It is a source of fibrinogen, factor VIII, factor XIII, and von Willebrand factor, which are essential for blood clotting.
- It can be used to treat a variety of bleeding disorders.
- It is generally well-tolerated by patients.
Other Blood Components
In addition to the blood components described above, there are a number of other blood products that can be used for transfusion. These blood products include:
- Specific immunoglobulins: Specific immunoglobulin can be obtained from donors with high titres of antibody for example anti-hepatitis B or anti-rubella. These are antibodies that are used to treat certain infections and autoimmune disorders.
- Granulocyte concentrates: are prepared as buffy coats or on blood cell separators from normal healthy donors or from patients with CML. They have been used in patients with severe neutropenia who are not responding to antibiotic therapy.
- Human albumin solution (4.5%): Human albumin solution is prepared from human plasma and is a useful plasma volume expander when a sustained osmotic effect is required prior to the administration of blood. It is also used for fluid replacement in patients undergoing plasmapheresis and sometimes for fluid replacement in selected patients with hypoalbuminemia.
- Human albumin solution (20%) (salt-poor albumin): This blood component may be used in severe hypoalbuminemia when it is necessary to use a blood product with minimal electrolyte content. Principal indications for the use of this blood product are patients with nephrotic syndrome or liver failure.
- Freeze-dried factor VIII concentrate: This is a preparation of clotting factor VIII that is used to treat hemophilia A.
- Protein C concentrates: Protein C concentrate is used in severe sepsis with DIC to reduce thrombosis resulting from depletion of protein C.
- Freeze-dried factor IX-prothrombin complex concentrates: Mainly used for treating factor IX deficiency but are also used in patients with liver disease or in hemorrhage following overdose with oral anticoagulants or in patients with factor VIII inhibitors.
- Immunoglobulin: Pooled immunoglobulin is a valuable source of antibodies against common viruses. It is used in hypogammaglobulinemia for protection against viral and bacterial disease. This blood product may also be used in immune thrombocytopenia and other acquired immune disorders.
Specialized Blood Components for Unique Patient Needs
Irradiated Blood Products
Irradiated blood components are crucial for preventing a rare but highly lethal complication called Transfusion-Associated Graft-versus-Host Disease (TA-GVHD). This disease occurs when donor lymphocytes proliferate in the recipient and attack the recipient’s tissues, which cannot mount a counter-attack due to a compromised immune system or high genetic similarity between donor and recipient. Gamma irradiation inactivates the donor T-lymphocytes, rendering them incapable of division and proliferation without significantly damaging the blood’s other components (RBCs or platelets).
Storage and Transportation Conditions of Blood Product
Same as the non-irradiated component (e.g., 1°C to 6°C for RBCs; 20°C to 24°C for Platelets). The shelf life is original expiry date OR 28 days from the date of irradiation, whichever is sooner. This is because irradiation causes subtle changes that lead to a faster accumulation of potassium and slight hemolysis over time.
Indications for Use of Blood Product
Patients at risk of TA-GVHD, including
- Recipients of blood from first or second-degree relatives.
- Immunocompromised patients (e.g., bone marrow transplant recipients, patients with congenital immunodeficiencies, or patients undergoing high-dose chemotherapy).
- All intrauterine transfusions and neonate exchange transfusions.
- Patients receiving HLA-matched platelets.
Benefits of Irradiated Blood Products
The primary and essential benefit is the prevention of TA-GVHD, which has a mortality rate often exceeding 90%.
Washed Red Blood Cells (RBCs)
Washing is a laboratory procedure where red blood cells are separated from the surrounding plasma and then repeatedly washed (often three times) with sterile normal saline to remove virtually all traces of plasma proteins, electrolytes, and preservative solution.
The primary indication is for patients who have had severe, recurrent allergic or anaphylactic reactions due to plasma proteins. This is most critical for patients with Selective Immunoglobulin A (IgA) Deficiency who have developed anti-IgA antibodies. If these patients are transfused with standard blood containing donor IgA, it can trigger a life-threatening anaphylactic shock.
Storage and Transportation Conditions of Blood Product
1°C to 6°C. The shelf life significantly reduces to 24 hours from the time the unit was washed (if stored at 1°C to 6°C). This rapid expiration is because the washing process breaks the closed-system sterility of the original bag, increasing the risk of bacterial contamination.
Indications for Use of Blood Product
- Patients with documented IgA deficiency and anti-IgA antibodies to prevent anaphylaxis.
- Patients with a history of recurrent, severe allergic reactions (like anaphylaxis or severe urticaria) not prevented by pre-medication.
- Neonates or patients with renal failure requiring blood, as washing removes the accumulated extracellular potassium that is present in stored red blood cell units.
Benefits
- Prevents life-threatening anaphylactic reactions in plasma-sensitive patients (e.g., IgA deficient patients).
- Reduces the potassium load, making it safer for patients who cannot excrete potassium effectively (e.g., neonates and those with renal impairment).
Compatibility and Pre-Transfusion Testing
Compatibility testing is a multi-step process that ensures the patient’s immune system will not attack the transfused red blood cells (RBCs). The three main pillars of compatibility are: ABO/Rh Typing, Antibody Screening, and Crossmatching.
ABO and RhD Typing (Group)
This is the foundational step, determining the presence or absence of the two major antigen systems.
| Test Component | Method | Purpose |
| ABO Typing | Forward Grouping: Testing the patient’s RBCs for A and B antigens. Reverse Grouping: Testing the patient’s plasma for anti-A and anti-B antibodies. | To confirm the patient’s blood type (A, B, AB, or O). The forward and reverse results must agree. |
| RhD Typing (D Antigen) | Testing the patient’s RBCs for the presence of the D antigen (determining Rh-positive or Rh-negative). | To ensure Rh-negative patients receive Rh-negative RBCs to prevent the formation of the highly reactive anti-D antibody. |
Antibody Screen (Screen)
The antibody screen is a critical safety step used to detect “unexpected” or alloantibodies in the patient’s plasma. These antibodies are usually IgG-class and are formed due to prior exposure to foreign RBC antigens, typically through previous transfusions or pregnancy.
If the screen is positive, the lab must perform an Antibody Identification panel. This delays the transfusion but is necessary to select units that are negative for the corresponding antigen, thereby preventing a delayed but serious hemolytic transfusion reaction.
Crossmatch (Compatibility Test)
The crossmatch is the final compatibility check that simulates the transfusion in vitro (in a test tube or other platform). It is the ultimate safeguard against incompatibility.
- Major Crossmatch: The most critical step in compatibility testing. Recipient plasma is mixed with a sample of the Donor’s Red Blood Cells. If agglutination (clumping) or hemolysis occurs, the unit is incompatible and cannot be used.
- Electronic (Computer) Crossmatch: Used when the patient has no history of unexpected antibodies and the current antibody screen is negative. A validated computer system checks the ABO/Rh compatibility between the patient and donor records, allowing blood to be issued quickly and safely.
- Serological (Manual) Crossmatch: Performed when the patient has a history of or current unexpected antibodies. This involves the full incubation and antihuman globulin steps to ensure the unit is fully compatible.
Specialized Compatibility Considerations
Beyond the main ABO/Rh matching, two other factors require careful selection for high-risk patients:
Cytomegalovirus (CMV) Status
CMV is a common virus that remains latent in the donor’s leukocytes (white blood cells).
- Patient Risk: CMV-negative patients who are highly immunocompromised (e.g., premature infants, hematopoietic stem cell transplant recipients, or HIV patients with low CD4 counts) are at high risk of fatal primary infection if transfused with CMV-positive blood.
- CMV-Safe Strategies: For these high-risk groups, blood components must be CMV-safe, achieved by:
- Using CMV Seronegative blood (donors who test negative for CMV antibodies).
- Using Leukodepleted blood products, which are considered equally effective as removing the leukocytes effectively removes the virus-carrying cells.
Sample Validity (The 72-Hour Rule)
To prevent serious errors, the time frame for which a pre-transfusion sample is considered valid is restricted, particularly for patients at high risk of developing new antibodies.
- 72-Hour Rule: If a patient has been transfused or pregnant within the last three months, their blood sample is only valid for 72 hours (3 days).
- Rationale: Transfusion or pregnancy is a major immune stimulus that can trigger the formation of a new, clinically significant alloantibody. If a transfusion were given using an older sample, that new antibody might be missed, leading to a severe HTR. A new sample must be drawn and tested after the 72-hour window.
Frequently Asked Questions (FAQs)
What is the main difference between Whole Blood and Packed Red Blood Cells (PRBCs)?
Whole Blood contains all blood components (RBCs, WBCs, platelets, and plasma) and is mainly used for massive blood loss when all blood components are needed. PRBCs are concentrated red cells with most of the plasma removed. PRBCs are the blood product of choice for treating chronic or symptomatic anemia because they effectively increase oxygen-carrying capacity with less volume.
Why is it necessary to remove White Blood Cells (Leukodepletion) from donated blood?
Leukodepletion removes white blood cells (leukocytes) to significantly reduce the risk of certain adverse transfusion reactions. These include Febrile Non-Hemolytic Transfusion Reactions (FNHTRs) and the transmission of cell-associated viruses like Cytomegalovirus (CMV), which is critical for immunocompromised patients.
Are all blood components matched for the patient’s ABO and Rh blood type?
Packed Red Blood Cells (PRBCs) must be meticulously matched for both ABO and Rh types to prevent life-threatening hemolytic reactions. Plasma and Cryoprecipitate blood products contain antibodies, so while they don’t carry the same risk as RBCs, ABO compatibility is still important for plasma. Platelets contain minimal plasma but are often ideally ABO-matched, though not always mandatory for life-saving transfusions.
What is “Irradiated Blood” and who needs it?
Irradiated blood product has been treated with a controlled dose of radiation to inactivate donor T-lymphocytes (a type of white blood cell). This prevents the donor’s immune cells from attacking the recipient’s tissues, a fatal condition called Transfusion-Associated Graft-versus-Host Disease (TA-GVHD). This blood product is required for highly immunocompromised patients, newborns, and recipients of blood from a genetically related donor.
Why is Fresh Frozen Plasma (FFP) frozen so quickly after collection?
FFP must be frozen rapidly (typically within 8 hours) to ensure the preservation of the highly labile clotting factors, specifically Factor V and Factor VIII, which degrade quickly at room temperature. These factors are essential for treating severe bleeding and coagulation factor deficiencies.
Glossary of Medical Terms
- Apheresis: A medical technology where the blood of a donor or patient is passed through an apparatus that separates out one particular blood component (e.g., platelets or plasma), returning the remainder of the blood to the donor/patient.
- Cryoprecipitate: A frozen blood component prepared from Fresh Frozen Plasma (FFP) that is rich in Fibrinogen, Factor VIII, Factor XIII, and von Willebrand Factor. Used primarily to treat deficiencies in these factors.
- DIC (Disseminated Intravascular Coagulation): A life-threatening condition where proteins that control blood clotting become hyperactive, leading to the formation of small clots throughout the bloodstream, which ultimately depletes clotting factors and causes massive bleeding.
- Dysfibrinogenemia: A condition where the body produces fibrinogen, but it is structurally or functionally abnormal, impairing normal blood clot formation.
- FFP (Fresh Frozen Plasma): The fluid portion of blood (plasma) that is frozen rapidly (within 8 hours of collection) to preserve labile clotting factors (like Factor V and Factor VIII). This blood product is used to replace coagulation factors in bleeding patients.
- Fibrinogen: A soluble protein in the plasma that is converted into Fibrin during the clotting process, forming the meshwork of a blood clot. Also known as Coagulation Factor I.
- FNHTRs (Febrile Non-Hemolytic Transfusion Reactions): A common, non-life-threatening adverse reaction characterized by fever and/or chills, typically caused by the recipient’s antibodies reacting to residual donor Leukocytes (white blood cells) or cytokines in the blood product.
- Leukodepletion: The process of filtering out or removing white blood cells (leukocytes) from a blood product. This is done to reduce the risk of FNHTRs, TRALI, and the transmission of cell-associated viruses like CMV.
- PRBCs (Packed Red Blood Cells): Red blood cells separated from the plasma and a portion of the white blood cells. This is the most common blood component transfused to increase the oxygen-carrying capacity of the blood.
- TA-GVHD (Transfusion-Associated Graft-versus-Host Disease): A rare but highly lethal complication where immunocompetent donor T-lymphocytes proliferate in the recipient and attack the recipient’s tissues. Prevention is achieved by Irradiating blood products.
- Thrombocytopenia: A condition characterized by an abnormally low number of platelets (thrombocytes) in the blood, often leading to impaired clotting and increased risk of bleeding.
- TRALI (Transfusion-Related Acute Lung Injury): An acute, life-threatening syndrome of non-cardiogenic pulmonary edema (fluid in the lungs) occurring during or shortly after blood transfusion.
- Von Willebrand Disease: The most common inherited bleeding disorder, caused by a deficiency or defect in the von Willebrand Factor protein, which is necessary for platelet adhesion and protecting Factor VIII.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Dean L. Blood Groups and Red Cell Antigens [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2005. Chapter 5, The ABO blood group.
- Yamamoto F. ABO+logy: Bioscience of the ABO Blood Groups for High School/College Students & Adults (2023).
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Lotterman S, Sharma S. Blood Transfusion. [Updated 2023 Jun 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499824/
- Open Resources for Nursing (Open RN); Ernstmeyer K, Christman E, editors. Nursing Advanced Skills [Internet]. Eau Claire (WI): Chippewa Valley Technical College; 2023. Chapter 3 Administer Blood Products. Available from: https://www.ncbi.nlm.nih.gov/books/NBK594497/
- Basu, D., & Kulkarni, R. (2014). Overview of blood components and their preparation. Indian journal of anaesthesia, 58(5), 529–537. https://doi.org/10.4103/0019-5049.144647
- Raval, J. S., Griggs, J. R., & Fleg, A. (2020). Blood Product Transfusion in Adults: Indications, Adverse Reactions, and Modifications. American family physician, 102(1), 30–38.