Introduction
The D-dimer test is a rapid and widely used diagnostic tool primarily for its high negative predictive value, meaning a normal D-dimer level can effectively rule out acute thrombotic events like deep vein thrombosis (DVT) and pulmonary embolism (PE) in low-to-moderate risk patients. Elevated D-dimer levels, however, require further investigation as they can indicate thrombosis or other conditions such as infection, inflammation, trauma, or pregnancy.
Blood coagulation is a vital physiological process that prevents excessive bleeding following vascular injury. It involves a complex cascade of events leading to the formation of a fibrin clot. While essential for hemostasis, uncontrolled or inappropriate clot formation (thrombosis) can lead to life-threatening conditions such as deep vein thrombosis (DVT), pulmonary embolism (PE), stroke, and myocardial infarction.
The fibrinolytic system acts as a counterbalance to coagulation, responsible for the breakdown of fibrin clots. D-dimer is a specific degradation product of cross-linked fibrin. It is formed when plasmin, the primary enzyme of fibrinolysis, breaks down a fibrin clot that has been stabilized by Factor XIIIa. Therefore, the presence of D-dimer in the blood indicates that clot formation and subsequent clot breakdown have occurred.
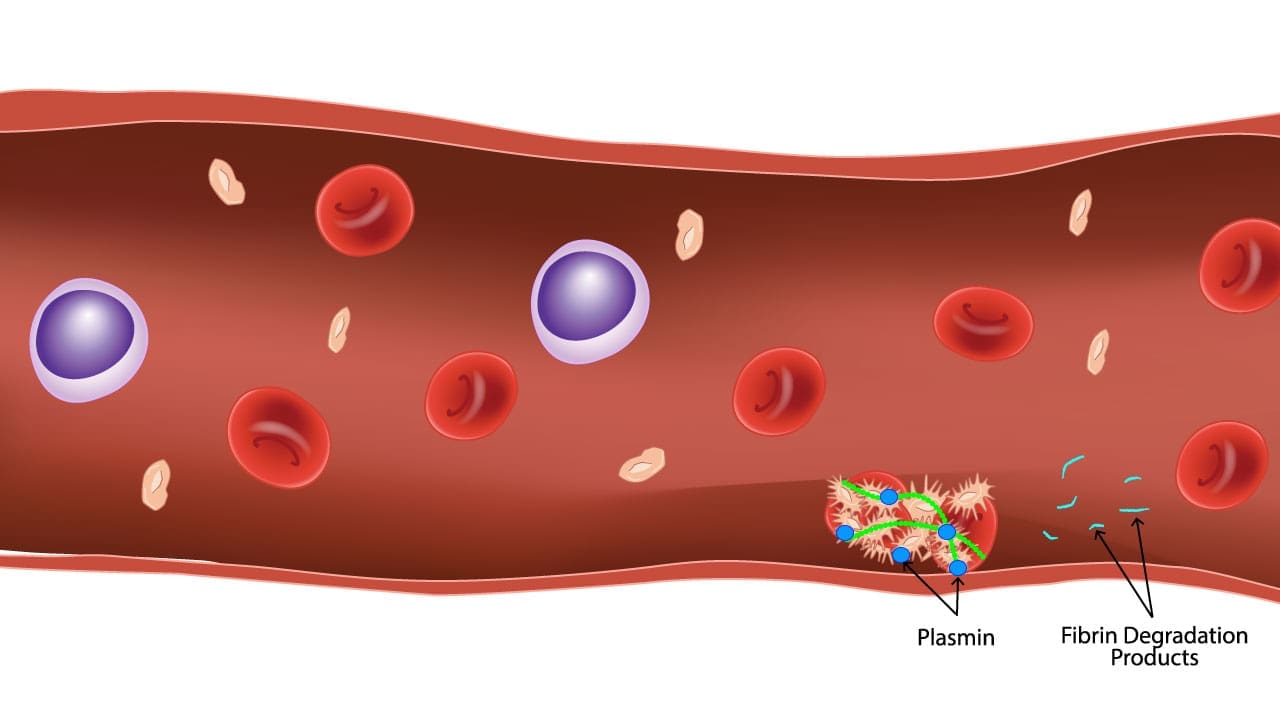
Fibrinolysis in action! This image illustrates the dissolution of a blood clot by plasmin. The fibrin strands, which forms the clot, is broken down into smaller fragments called fibrin degradation products (FDPs) including D-dimer. This regulated process ensures the clot doesn’t outstay its welcome, promoting smooth blood flow and tissue healing.
Principle of the Test
The quantitative D-dimer test often employs an immunoturbidimetric method. This principle is based on the antigen-antibody reaction and subsequent measurement of turbidity.
- Antigen-Antibody Reaction: The patient’s plasma sample, containing D-dimer (the antigen), is mixed with a reagent containing specific latex particles coated with monoclonal antibodies directed against D-dimer.
- Agglutination: If D-dimer is present in the sample, it binds to the antibodies on the latex particles, causing the particles to agglutinate (clump together). The degree of agglutination is directly proportional to the concentration of D-dimer in the sample.
- Turbidity Measurement: As agglutination occurs, the turbidity (cloudiness) of the reaction mixture increases.
- Spectrophotometric Detection: An automated analyzer passes a light beam through the sample and measures the decrease in light transmission (or increase in absorbance) due to the increased turbidity.
- Quantification: The change in absorbance over time is measured kinetically or at an endpoint. This change is then compared to a standard curve generated from known D-dimer concentrations, allowing for the accurate quantification of D-dimer in the patient’s sample.
Materials

- Blood collected in a light blue top sodium citrate tube.
- Automated Coagulation Analyzer.
- D-dimer reagent kit specific to the analyzer (typically includes latex particles coated with anti-D-dimer antibodies, buffer, diluent).
- D-dimer calibrators.
- D-dimer controls (low, normal, high pathological levels).
- Deionized water.
- Centrifuge (refrigerated, if available, for plasma preparation).
- Pipettes.
- Disposable pipette tips.
- Vortex mixer.
- Gloves, lab coats, safety glasses (PPE).
Methodology
Specimen Collection and Preparation
- Centrifuge the blood sample at 1500-2000 x g for 10-15 minutes at room temperature (18-25°C) to obtain platelet-poor plasma (PPP) within 1 hour of sample collection. *For optimal results, especially if testing is delayed: Re-centrifuge the plasma at 2500 x g for 10 minutes to ensure minimal platelet contamination.
- Carefully transfer the supernatant plasma using a plastic pipette into a clean, labeled secondary plastic tube (e.g., cryovial or aliquot tube), ensuring not to transfer any cellular elements.
- Analyze plasma immediately.
- If analysis is delayed, plasma can be stored at 2-8°C for up to 4-8 hours (check specific kit insert for exact stability).
- For longer storage, freeze plasma at -20°C for up to 1-2 weeks or at -70°C for up to 6 months. Thaw rapidly in a 37°C water bath just before testing and mix gently. Do not refreeze thawed samples.
General Automated Analyzer Method
(Please refer to manufacturer’s instructions for specific method)
- Power on the automated coagulation analyzer according to the manufacturer’s instructions.
- Perform any required daily maintenance, such as cleaning probes, checking fluid levels, and running system checks.
- Prepare D-dimer reagents as per the manufacturer’s instructions (e.g., reconstitution of lyophilized reagents, mixing components) and load into the analyzer.
- Perform calibration using the D-dimer calibrators. The analyzer will create a standard curve. *Calibration should be performed after reagent lot changes, major instrument maintenance, or as recommended by the manufacturer.
- Run D-dimer control samples (low, normal, high pathological) at the beginning of each shift, after calibration, and after any significant instrument troubleshooting.
- Plot QC results on a Levey-Jennings chart. Ensure all QC values fall within the acceptable range. If QC is out of range, troubleshoot and re-run.
- Load prepared patient plasma samples onto the analyzer’s sample rack.
- Review the results generated by the analyzer.
Common Troubleshooting
- Out-of-range QC
- Check reagent expiry and integrity.
- Ensure proper reagent mixing and reconstitution.
- Verify calibrator integrity and correct values.
- Perform instrument maintenance (e.g., probe cleaning, fluid checks).
- Re-run QC with fresh reagents/controls.
- Hemolysis, Icterus, Lipemia (HIL)
- Grossly hemolyzed, icteric, or lipemic samples can interfere with spectrophotometric assays. The analyzer may flag these samples.
- If possible, request a new sample. If not, results must be interpreted with caution and a note added.
- Fibrin Clots in Sample
- Presence of fibrin clots in the plasma indicates incomplete mixing during collection or prolonged delay before centrifugation. This can consume D-dimer and give falsely low results.
- Always inspect samples for clots before analysis. Reject clotted samples and request a redraw.
- Incorrect Fill Volume
- Under-filled or over-filled citrate tubes alter the blood-to-anticoagulant ratio, leading to erroneous results. Reject and redraw.
Interpretation of Results
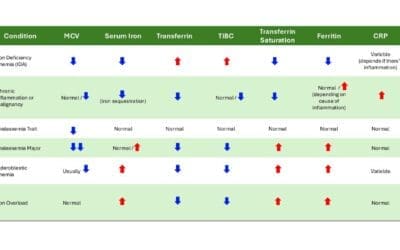
D-dimer results are typically reported in units of FEU (Fibrinogen Equivalent Units) or ng/mL. It’s crucial to know the specific reference range for the assay and instrument used, as ranges can vary slightly between manufacturers.
Normal Reference Range
The normal reference range of D-dimer is < 500 ng/mL FEU (or < 0.50 mg/L FEU) for adults under 50 years of age. Some guidelines suggest an age-adjusted D-dimer cutoff (Age x 10 ng/mL for patients over 50 years old) to improve specificity and reduce false positives in older individuals.
Clinical Interpretation
Normal D-dimer Level (e.g., < 500 ng/mL FEU)
In a patient with a low or intermediate pre-test probability of DVT or PE, a normal D-dimer result has a very high negative predictive value and effectively rules out these conditions. This allows for safe withholding of anticoagulant therapy and avoids unnecessary imaging studies.
It generally suggests that significant active thrombosis and fibrinolysis are NOT occurring.
Elevated D-dimer Level (e.g., > 500 ng/mL FEU)
Elevated D-dimer test result Indicates that fibrin formation and breakdown are occurring at an increased rate. An elevated D-dimer is non-specific and does not definitively diagnose a thrombotic event. It merely suggests the need for further investigation.
Clinical conditions associated with elevated D-dimer
- Thrombotic Events: Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), Disseminated Intravascular Coagulation (DIC), Stroke, Myocardial Infarction.
- Physiological States: Pregnancy (especially in the third trimester and postpartum), advanced age.
- Inflammatory/Infectious Conditions: Sepsis, severe infections, inflammatory bowel disease.
- Other Medical Conditions: Cancer, liver disease, kidney disease, recent surgery, trauma, extensive burns, acute aortic dissection, sickle cell crisis.
- Recent Bleeding or Hematoma: Large hematomas can also lead to elevated D-dimer.
Frequently Asked Questions (FAQs)
What infections cause high D-dimer?
Elevated D-dimer levels are commonly seen in various infectious conditions due to the activation of coagulation and fibrinolysis that often accompanies systemic inflammation and immune responses.
Most notably, sepsis and severe infections of any kind frequently lead to a significant rise in D-dimer, as the body’s generalized inflammatory state can trigger widespread microclot formation and subsequent breakdown.
Specific infections like pneumonia and more recently, COVID-19, particularly in severe cases requiring hospitalization or intensive care, have been well-documented to cause substantial D-dimer elevations, reflecting the associated coagulopathy and increased risk of thrombotic complications in these patients.
What if my D-dimer is high but no clot?
It’s quite common to have an elevated D-dimer level without an active blood clot. While D-dimer is an excellent test for ruling out a clot (a normal D-dimer strongly suggests no clot), its specificity is low, meaning many conditions other than a blood clot can cause it to be high. These non-thrombotic causes include physiological states like pregnancy (especially in later stages), advanced age (D-dimer levels naturally increase with age), and even strenuous physical activity.
Furthermore, various medical conditions can activate the coagulation and fibrinolytic systems, leading to elevated D-dimer without necessarily forming a new clot. These include infections (such as sepsis, pneumonia, or COVID-19), inflammatory conditions (like autoimmune diseases such as rheumatoid arthritis), cancer (due to increased procoagulant activity associated with malignancies), recent surgery or trauma, liver disease, and heart disease (e.g., heart failure, myocardial infarction).
Therefore, an elevated D-dimer always needs to be interpreted in conjunction with a patient’s clinical symptoms, medical history, and other diagnostic tests to determine the true underlying cause.
What cancers cause high D-dimer?
Many types of cancers can lead to elevated D-dimer levels, reflecting the complex interplay between cancer and the body’s coagulation and fibrinolytic systems. Common malignancies strongly associated with high D-dimer include lung cancer, gastrointestinal cancers (e.g., colorectal, pancreatic, gastric), breast cancer, prostate cancer, and gynecological cancers (e.g., ovarian, cervical, endometrial).
This elevation often correlates with advanced tumor stage, metastatic disease, and a poorer prognosis, even in the absence of a confirmed blood clot. The mechanisms behind this involve the tumor cells themselves producing procoagulant factors, stimulating inflammatory responses, and altering the vascular environment, all of which contribute to a state of hypercoagulability and increased fibrin turnover, thus generating more D-dimer.
Can exercise cause elevated D-dimer?
Yes, strenuous or intense physical exercise can indeed cause a temporary elevation in D-dimer levels. This is due to the activation of both the coagulation and fibrinolytic systems that occur during significant exertion.
When you engage in intense exercise, your body responds by releasing substances like adrenaline (catecholamines) and tissue plasminogen activator (t-PA) from endothelial cells. Adrenaline can promote some aspects of coagulation, while t-PA is a key enzyme in fibrinolysis (clot breakdown). The combination of these activities results in the production of D-dimer. While this is a physiological response and usually not indicative of an active pathological clot in healthy individuals, do take it into consideration when interpreting D-dimer results in individuals who have recently undergone strenuous exercise.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- Bain BJ, Bates I, Laffan MA. Dacie and Lewis Practical Haematology: Expert Consult: Online and Print 12th Edition (Elsevier). 2016.
- Saba HI, Roberts HR. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management 1st Edition (Wiley-Blackwell). 2014.
- Bounds EJ, Kok SJ. D Dimer. [Updated 2023 Aug 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431064/
- Imberti D. (2007). D-dimer testing: advantages and limitations in emergency medicine for managing acute venous thromboembolism. Internal and emergency medicine, 2(1), 70–71. https://doi.org/10.1007/s11739-007-0020-3