TL;DR
Shwachman-Diamond syndrome or SDS is a rare, autosomal recessive genetic disorder caused by a mutation in the SBDS gene on chromosome 7.
- Cause ▾: Shwachman-Diamond Syndrome (SDS) is a rare, inherited genetic disorder. It is an autosomal recessive condition caused by a mutation in the SBDS gene, which is critical for proper cellular function and ribosome formation.
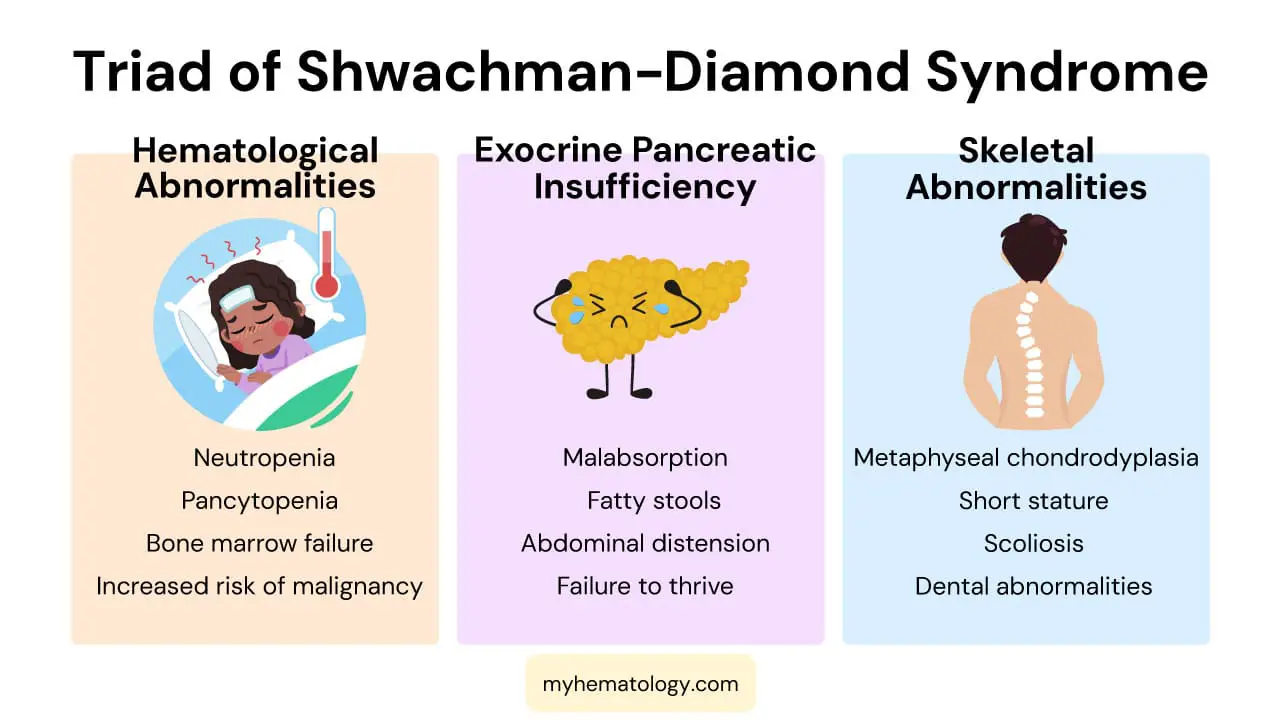
- Symptoms ▾: The syndrome presents as a classic triad of symptoms: exocrine pancreatic insufficiency, hematological abnormalities (especially neutropenia, which increases the risk of recurrent infections), and skeletal abnormalities like metaphyseal chondrodysplasia.
- Laboratory Investigations ▾: A complete blood count (CBC) will often show persistent or intermittent neutropenia. Pancreatic function is assessed with a fecal elastase-1 test, which will show markedly reduced levels. The definitive diagnosis is confirmed through genetic sequencing to identify the SBDS gene mutation.
- Treatment and Management ▾: The treatment is primarily supportive and multidisciplinary. Pancreatic enzyme replacement therapy (PERT) is used to manage gastrointestinal symptoms. Hematological issues are treated with granulocyte colony-stimulating factor (G-CSF) to address severe neutropenia, and in severe cases, hematopoietic stem cell transplantation (HSCT) may be required.
*Click ▾ for more information
Introduction to SDS
Shwachman-Diamond syndrome or SDS is a rare, autosomal recessive genetic disorder. It is characterized by a triad of key features: hematological abnormalities, pancreatic exocrine insufficiency, and skeletal dysplasia.
Shwachman-Diamond syndrome (SDS) is a multisystem disorder that affects multiple organs and systems throughout the body. The condition presents significant diagnostic and management challenges. Understanding the underlying pathophysiology and clinical manifestations is crucial for providing effective care and improving patient outcomes.
Pathophysiology: The Role of the SBDS Gene
The primary cause of Shwachman-Diamond syndrome (SDS) is a mutation in the SBDS gene, located on chromosome 7q11. The SBDS gene provides instructions for creating the SBDS protein. This protein plays a critical, yet not fully understood, role in cellular processes, most notably in ribosome biogenesis and function.
Ribosomes are the cellular machinery responsible for protein synthesis. They are composed of two subunits, a large and a small one. The SBDS protein is essential for the maturation and assembly of the 60S ribosomal subunit. A deficiency or dysfunction of the SBDS protein disrupts this process. This leads to a defect in ribosome biogenesis. The impaired ribosomal function affects protein translation, particularly in rapidly dividing cells. This is thought to be the core mechanism behind the clinical features of Shwachman-Diamond syndrome (SDS), especially the hematopoietic and pancreatic deficiencies.
Research suggests that the SBDS protein may also be involved in stress responses and mitotic spindle formation. These broader cellular roles might explain the wide spectrum of clinical abnormalities observed in Shwachman-Diamond syndrome (SDS). The severity of the phenotype can vary. This is likely due to the specific type of mutation and the residual function of the SBDS protein.
Clinical Manifestations & Symptoms of Shwachman-Diamond Syndrome (SDS)
Shwachman-Diamond syndrome (SDS) is a multisystem disorder with diverse clinical features. The presentation and severity can vary among individuals.
Hematological Abnormalities
Hematological issues are present in nearly all patients with Shwachman-Diamond syndrome (SDS).
- Neutropenia: A consistent or intermittent decrease in neutrophils, increasing the risk of recurrent bacterial infections.
- Pancytopenia: Lower-than-normal counts of all three types of blood cells (red blood cells, white blood cells, and platelets).
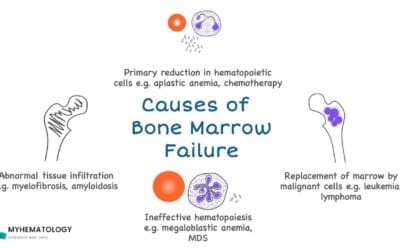
- Bone Marrow Failure: A hallmark of Shwachman-Diamond syndrome (SDS) is hypocellular or hypoplastic bone marrow. This reflects the defect in hematopoietic stem cell function. Over time, some patients may develop severe aplastic anemia, requiring significant medical intervention.
- Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML): Patients with Shwachman-Diamond syndrome (SDS) have a significantly increased risk of developing MDS and AML. The cumulative incidence is estimated to be between 20% and 30% by the age of 30. Regular hematologic monitoring is essential for early detection of these clonal evolution events.
Pancreatic Exocrine Insufficiency
This is a classic feature of Shwachman-Diamond syndrome (SDS), present in almost 100% of affected individuals in early childhood.
- Malabsorption: The pancreas fails to produce digestive enzymes (e.g., lipase, amylase, and proteases). This results in severe malabsorption of fats and fat-soluble vitamins (A, D, E, K).
- Gastrointestinal Symptoms: Patients typically present with symptoms such as steatorrhea (fatty stools), abdominal distension, and failure to thrive.
Skeletal Abnormalities
Skeletal dysplasia is a consistent finding in Shwachman-Diamond syndrome (SDS), affecting approximately 60–80% of patients.
- Metaphyseal Chondrodysplasia: This is the most characteristic skeletal finding. It affects the metaphyses of long bones, leading to abnormal bone growth.
- Short Stature: Most individuals with Shwachman-Diamond syndrome (SDS) have short stature due to a combination of skeletal dysplasia and malnutrition from malabsorption.
- Other Skeletal Issues: Patients may also experience a variety of other orthopedic issues, including rib cage abnormalities, scoliosis, and dental anomalies. The skeletal issues can progress with age, causing functional limitations.
Other Systemic Features
Shwachman-Diamond syndrome (SDS) can affect other organs and systems.
- Growth and Development: Beyond short stature, patients often experience developmental delays, including cognitive and motor delays.
- Hepatic Dysfunction: Mild liver dysfunction and hepatomegaly are common, typically resolving with age.
- Immune Dysfunction: Beyond neutropenia, some patients may have compromised immune function, including T-cell and B-cell deficiencies, increasing susceptibility to infections.
Laboratory Investigations and Diagnosis of Shwachman-Diamond Syndrome (SDS)
The diagnosis of Shwachman-Diamond syndrome (SDS) is a process that integrates clinical presentation, laboratory findings, and genetic confirmation.
Diagnostic Criteria
Diagnosis should be considered in any child with a combination of two or more of the following:
- Unexplained neutropenia or other cytopenias
- Clinical suspicion of pancreatic exocrine insufficiency
- Skeletal abnormalities consistent with metaphyseal chondrodysplasia
Hematological Studies
- Complete Blood Count (CBC) with differential: To assess for neutropenia, anemia, and thrombocytopenia.
- Bone marrow aspiration and biopsy: To evaluate for hypocellularity or signs of MDS/AML.
Gastrointestinal Studies
- Fecal Elastase-1: A non-invasive and sensitive test for pancreatic exocrine function. Markedly reduced levels of fecal elastase-1 (often less than 200μg/g of stool), indicating pancreatic exocrine insufficiency.
- Serum trypsinogen levels: Very low to undetectable serum trypsinogen levels. This is a direct result of the pancreatic exocrine insufficiency, as the pancreas is unable to produce and secrete this enzyme into the bloodstream.
Genetic Testing
Genetic sequencing of the SBDS gene is the gold standard for definitive diagnosis. The definitive diagnosis is established by identifying biallelic pathogenic variants in the SBDS gene through genetic sequencing.
Clinical Management and Treatment of Shwachman-Diamond Syndrome (SDS)
Management of Shwachman-Diamond syndrome (SDS) is complex and requires a multidisciplinary approach involving hematologists, gastroenterologists, nutritionists, orthopedic specialists, and genetic counselors.
Hematology
- Neutropenia: Granulocyte colony-stimulating factor (G-CSF) can be used to manage severe, symptomatic neutropenia.
- Bone Marrow Failure: For patients with severe bone marrow failure or advanced MDS/AML, allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative option. HSCT is a high-risk procedure but can correct the hematopoietic defects.
- Surveillance: Patients require lifelong hematological monitoring with regular blood counts and annual bone marrow evaluations to screen for MDS/AML.
Gastroenterology and Nutrition
- Pancreatic Enzyme Replacement Therapy (PERT): PERT is the cornerstone of treatment for pancreatic insufficiency. It involves taking pancreatic enzyme supplements with meals to aid digestion and nutrient absorption.
- Nutritional Support: A high-calorie, low-fat diet may be recommended. Supplementation with fat-soluble vitamins (A, D, E, K) is essential. Regular monitoring of growth and nutritional status is critical.
Orthopedic Management
Skeletal abnormalities are managed symptomatically. This may include physical therapy, bracing for scoliosis, and, in some cases, orthopedic surgery to correct severe deformities.
Prognosis and Long-Term Outlook
The prognosis for individuals with Shwachman-Diamond syndrome (SDS) has significantly improved with advancements in supportive care and HSCT. The most significant cause of morbidity and mortality is the progression to MDS/AML and severe infections. Regular follow-up and monitoring are essential. Continued research into the molecular mechanisms of Shwachman-Diamond syndrome (SDS) offers hope for more targeted therapies in the future.
Frequently Asked Questions (FAQs)
What is the average life expectancy for someone with Shwachman-Diamond syndrome (SDS)?
The life expectancy for individuals with Shwachman-Diamond syndrome (SDS) has improved significantly. While it remains variable, the main risks are related to bone marrow failure and the development of MDS/AML. Early diagnosis and proactive management, including potential hematopoietic stem cell transplantation, are key to improving long-term outcomes.
Is Shwachman-Diamond syndrome (SDS) a curable condition?
The hematological features of Shwachman-Diamond syndrome (SDS) can be cured by allogeneic hematopoietic stem cell transplantation (HSCT), which replaces the defective hematopoietic stem cells. However, this procedure does not correct the skeletal or pancreatic issues. There is currently no cure for the syndrome itself.
Is genetic testing required for diagnosis?
Yes, genetic testing for mutations in the SBDS gene is considered the gold standard for confirming a diagnosis of Shwachman-Diamond syndrome (SDS). While clinical features are highly suggestive, a definitive diagnosis is based on identifying the underlying genetic cause.
Can Shwachman-Diamond syndrome (SDS) be detected before birth?
Prenatal diagnosis is possible for families with a known history of Shwachman-Diamond syndrome (SDS). Genetic testing can be performed on chorionic villus sampling or amniocentesis to confirm the diagnosis in the fetus. Genetic counseling is highly recommended for families at risk.
Glossary
- Autosomal Recessive: A pattern of inheritance where an individual must inherit two copies of a mutated gene, one from each parent, to be affected by the disorder.
- Chondrodysplasia: A genetic disorder of bone and cartilage development, often resulting in skeletal abnormalities and short stature.
- Hematopoietic Stem Cell Transplantation (HSCT): A medical procedure that replaces a patient’s unhealthy bone marrow with healthy blood-forming stem cells from a donor.
- Myelodysplastic Syndrome (MDS): A group of cancers in which immature blood cells in the bone marrow do not mature properly and therefore do not become healthy blood cells. It is a precursor to AML.
- Neutropenia: An abnormally low count of neutrophils, a type of white blood cell crucial for fighting bacterial and fungal infections.
- Pancreatic Exocrine Insufficiency: A condition where the pancreas does not produce enough of the digestive enzymes needed to break down food.
- Ribosome Biogenesis: The process of making ribosomes, the cell’s protein synthesis factories.
- Steatorrhea: The presence of excess fat in feces, caused by malabsorption.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Farooqui SM, Ward R, Aziz M. Shwachman-Diamond Syndrome. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507866/
- Shimamura A. (2021). Molecular alterations governing predisposition to myelodysplastic syndromes: Insights from Shwachman-Diamond syndrome. Best practice & research. Clinical haematology, 34(1), 101252. https://doi.org/10.1016/j.beha.2021.101252
- Furutani, E., Liu, S., Galvin, A., Steltz, S., Malsch, M. M., Loveless, S. K., Mount, L., Larson, J. H., Queenan, K., Bertuch, A. A., Fleming, M. D., Gansner, J. M., Geddis, A. E., Hanna, R., Keel, S. B., Lau, B. W., Lipton, J. M., Lorsbach, R., Nakano, T. A., Vlachos, A., … Shimamura, A. (2022). Hematologic complications with age in Shwachman-Diamond syndrome. Blood advances, 6(1), 297–306. https://doi.org/10.1182/bloodadvances.2021005539
- Lawal, O. S., Mathur, N., Eapi, S., Chowdhury, R., & Malik, B. H. (2020). Liver and Cardiac Involvement in Shwachman-Diamond Syndrome: A Literature Review. Cureus, 12(1), e6676. https://doi.org/10.7759/cureus.6676
- Cull, A. H., Kent, D. G., & Warren, A. J. (2024). Emerging genetic technologies informing personalized medicine in Shwachman-Diamond syndrome and other inherited BMF disorders. Blood, 144(9), 931–939. https://doi.org/10.1182/blood.2023019986
- Han, X., Lu, S., Gu, C., Bian, Z., Xie, X., & Qiao, X. (2023). Clinical features, epidemiology, and treatment of Shwachman-Diamond syndrome: a systematic review. BMC pediatrics, 23(1), 503. https://doi.org/10.1186/s12887-023-04324-3
- Thompson, A. S., Giri, N., Gianferante, D. M., Jones, K., Savage, S. A., Alter, B. P., & McReynolds, L. J. (2022). Shwachman Diamond syndrome: narrow genotypic spectrum and variable clinical features. Pediatric research, 92(6), 1671–1680. https://doi.org/10.1038/s41390-022-02009-8
- Bezzerri, V., & Cipolli, M. (2019). Shwachman-Diamond Syndrome: Molecular Mechanisms and Current Perspectives. Molecular diagnosis & therapy, 23(2), 281–290. https://doi.org/10.1007/s40291-018-0368-2
- Reilly, C. R., & Shimamura, A. (2023). Predisposition to myeloid malignancies in Shwachman-Diamond syndrome: biological insights and clinical advances. Blood, 141(13), 1513–1523. https://doi.org/10.1182/blood.2022017739