TL;DR
Smoldering Multiple Myeloma (SMM) is an intermediate, asymptomatic stage between Monoclonal Gammopathy of Undetermined Significance (MGUS) (low-risk) and Active Multiple Myeloma (MM) (symptomatic, requiring treatment).
- Pathophysiology ▾: Smoldering Multiple Myeloma (SMM) is driven by the clonal proliferation of malignant plasma cells within the bone marrow. Its progression is fueled by accumulating genetic aberrations (e.g., high-risk translocations like t(4;14) or deletion of 17p) and the supportive signals from the bone marrow microenvironment.
- Clinical presentation ▾: Patients with SMM are asymptomatic. The diagnosis is always an incidental finding and must be confirmed by specific lab and imaging tests.
- Investigations ▾: Diagnosis requires Serum Protein Electrophoresis (SPEP), Serum Free Light Chain (SFLC) assay, and a Bone Marrow Biopsy with FISH analysis.
- Diagnosis (IMWG criteria) ▾: To be diagnosed with SMM, a patient must meet two criteria while strictly excluding the third:
- High Tumor Burden: Monoclonal protein (M-protein) ≥ 3 g/dL and/or Clonal Bone Marrow Plasma Cells (BMPC) 10% to 60%.
- Absence of Myeloma-Defining Events (MDEs): There must be no evidence of CRAB criteria (Hypercalcemia, Renal failure, Anemia, Bone lesions) or MM biomarkers (BMPC ≥ 60%, FLCr ≥ 100, > 1 focal lesion).
- Risk Stratification & Prognosis ▾: Management is entirely dependent on risk stratification. The main complication is the progression to Active MM, with an average risk of ~ 10% per year for the first five years.
- High-Risk SMM: Defined by markers (e.g., BMPC > 20% and/or M-protein ≥ 3 g/dL and/or FLCr ≥ 20) that predict a progression risk of > 50% within two years.
- Management ▾:
- Low/Intermediate-Risk Management: The standard of care is Active Surveillance (or “Watch and Wait”), involving regular blood tests and imaging, to monitor for progression.
- High-Risk Management: These patients are candidates for Early Intervention via clinical trials, often using combinations of new agents (e.g., Lenalidomide and Daratumumab) to delay or prevent the onset of symptomatic MM.
- Complications ▾: Key risks include Immunoparesis (suppression of normal antibodies, increasing infection risk) and accelerated Osteoporosis (increased fracture risk due to bone turnover dysregulation).
*Click ▾ for more information
Introduction
Smoldering Multiple Myeloma (SMM) is a critical, asymptomatic precursor to the aggressive blood cancer known as Multiple Myeloma (MM). It represents an intermediate stage in the disease continuum, sitting between the nearly benign condition Monoclonal Gammopathy of Undetermined Significance (MGUS) and symptomatic, active MM.
All three conditions – MGUS, Smoldering Multiple Myeloma (SMM), and MM – are characterized by the excessive production of abnormal plasma cells (clonal plasma cells) in the bone marrow and the presence of a monoclonal protein (M-protein) in the blood or urine. The difference lies primarily in the quantity of these abnormal cells and proteins, and the presence of symptoms.
Clinical Significance: The Need for Monitoring
The primary significance of an Smoldering Multiple Myeloma (SMM) diagnosis is the inherent risk of progression to active MM. Unlike MGUS, which has a low annual risk (around 1%), the risk of progression for standard Smoldering Multiple Myeloma (SMM) is approximately 10% per year for the first five years, dropping to 3% per year thereafter.
Because the condition is currently asymptomatic, the standard management for low-risk and intermediate-risk Smoldering Multiple Myeloma (SMM) is active surveillance (watchful waiting). This involves frequent blood tests, urine tests, and imaging to monitor for any sign of progression to symptomatic disease (i.e., the development of the CRAB criteria or other myeloma-defining events).
The goal of Smoldering Multiple Myeloma (SMM) management is to identify the few patients who will benefit from early treatment, while sparing the majority from the side effects of therapy they may never need.
Pathophysiology and Pathogenesis of Smoldering Multiple Myeloma
Smoldering Multiple Myeloma (SMM) is driven by the transformation and accumulation of malignant plasma cells in the bone marrow. Its pathogenesis is defined by three interconnected pillars: Clonal Plasma Cell Expansion, Molecular and Genetic Aberrations, and the Bone Marrow Microenvironment (BMM) Interaction.
Clonal Plasma Cell Expansion
The foundation of Smoldering Multiple Myeloma (SMM) is the excessive growth of a single clone of plasma cells. The malignant plasma cells originate from a post-germinal center B-lymphocyte that has undergone somatic hypermutation and class-switch recombination. This cell acquires an initial transforming genetic lesion.
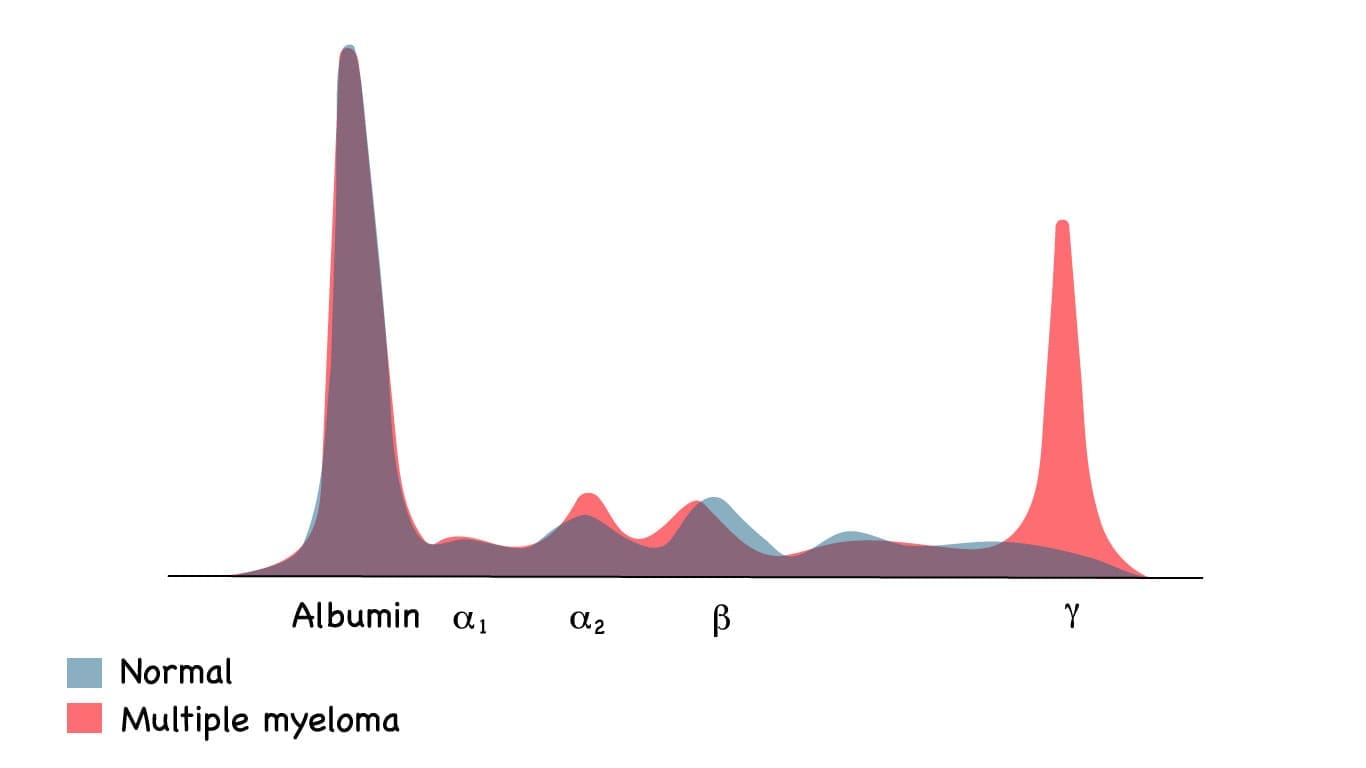
These clonal plasma cells produce and secrete vast quantities of a single, structurally identical immunoglobulin or immunoglobulin fragment, known as the monoclonal protein, M-protein, or paraprotein. The presence of this M-protein (IgG or IgA ≥ 3 g/dL) is a hallmark of Smoldering Multiple Myeloma (SMM).
By definition, Smoldering Multiple Myeloma (SMM) involves a substantial infiltration of clonal plasma cells in the bone marrow, ranging from 10% to 60% of all nucleated cells. This high burden increases the likelihood of interactions that drive disease progression compared to MGUS.
Molecular and Genetic Aberrations (Clonal Evolution)
Disease progression from MGUS to Smoldering Multiple Myeloma (SMM) to Active MM is a multi-step process characterized by the sequential accumulation of genetic abnormalities, a concept known as clonal evolution.
Primary Events (Occur Early)
These lesions are often found in MGUS and Smoldering Multiple Myeloma (SMM) patients and establish the initial malignant clone:
- Hyperdiploidy: This involves extra copies (trisomies) of odd-numbered chromosomes (e.g., 3, 5, 7, 9, 11, 15, 19, 21). These are generally associated with a slightly lower risk of progression compared to other lesions.
- Immunoglobulin Heavy Chain (IgH) Translocations: These translocations involve chromosome 14q32, which houses the IgH locus. t(11;14) is the most common translocation, involving cyclin D1. While often associated with Smoldering Multiple Myeloma (SMM), it can be a relatively stable abnormality.
Secondary and High-Risk Events (Drive Progression)
These aberrations are markers of genetic instability and are strongly associated with the rapid transition of Smoldering Multiple Myeloma (SMM) to active MM, placing patients into the “High-Risk SMM” category:
- del(17p): Deletion of the short arm of chromosome 17, which contains the tumor suppressor gene p53. Loss of p53 function leads to uncontrolled cell growth and resistance to apoptosis, severely accelerating disease progression.
- t(4;14): Translocation involving the fibroblast growth factor receptor (FGFR3) and the multiple myeloma SET domain (MMSET) gene. This is a high-risk factor promoting cell proliferation.
- t(14;16) and t(14;20): Less common but also associated with adverse outcomes.
The presence of any of these high-risk secondary events, even in an asymptomatic Smoldering Multiple Myeloma (SMM) patient, is a strong indicator of imminent progression.
Role of the Bone Marrow Microenvironment (BMM)
The BMM is a dynamic ecosystem that normally supports healthy cell function. In Smoldering Multiple Myeloma (SMM), the clonal plasma cells corrupt this environment, turning it into a protective niche that fosters their survival and proliferation.
- Cytokine Production: Abnormal plasma cells interact with bone marrow stromal cells, macrophages, and other cells, leading to the overproduction of cytokines and growth factors. Key examples include:
- Interleukin-6 (IL-6): A potent growth factor that acts as a survival and proliferation signal for the plasma cells.
- Hepatocyte Growth Factor (HGF): Promotes cell motility and contributes to drug resistance.
- Immune Dysfunction (Immunoparesis): The presence of the plasma cell clone suppresses the development and function of normal B cells, T cells, and antigen-presenting cells (like Dendritic Cells). This immunoparesis is a key feature of Smoldering Multiple Myeloma (SMM), leading to a functional immune deficiency characterized by low levels of unaffected polyclonal immunoglobulins. This suppression increases the patient’s susceptibility to serious infections, even before active MM develops.
- Osteoclast Activation (Pre-CRAB): Even without overt bone lesions (the ‘B’ in CRAB), early signaling changes in the BMM can lead to increased activity of osteoclasts (bone-resorbing cells) and suppressed activity of osteoblasts (bone-forming cells). This subtle imbalance primes the bone for the lytic lesions that characterize active MM.
Clinical Presentation of Smoldering Multiple Myeloma
The clinical presentation of Smoldering Multiple Myeloma (SMM) is characterized by a paradox: the presence of significant malignancy markers (high plasma cell burden, high M-protein) in a patient who feels entirely well. The clinical definition relies entirely on the absence of disease-related symptoms.
The Defining Feature: Asymptomatic Status
Smoldering Multiple Myeloma (SMM) is, by its most crucial diagnostic criterion, an asymptomatic disorder. Patients typically come to medical attention incidentally. Diagnosis often occurs when routine blood work (e.g., during an annual physical) reveals elevated total protein, prompting further investigation through tests like Serum Protein Electrophoresis (SPEP).
Patients do not experience the common “B symptoms” associated with many cancers (unexplained fever, night sweats, significant weight loss), nor do they suffer from organ damage or systemic complications that define active multiple myeloma.
The Absence of Myeloma-Defining Events (MDEs)
The absolute lack of end-organ damage is what classifies the disease as “smoldering” rather than “active.” If any of the following Myeloma-Defining Events (MDEs) are present, the patient is immediately reclassified and requires treatment.
The CRAB Criteria
The traditional MDEs are summarized by the CRAB acronym. SMM patients must show a complete absence of all these findings:
- C – Hypercalcemia: Abnormally high levels of calcium in the blood (serum calcium > 11 mg/dL or the upper limit of normal). This is a result of plasma cells stimulating excessive bone breakdown (osteoclast activity).
- R – Renal Insufficiency: Impairment of kidney function (serum creatinine > 2 mg/dL or creatinine clearance < 40 mL/min). This is often caused by the deposition of M-protein (light chains) in the renal tubules.
- A – Anemia: Low red blood cell count (Hemoglobin < 10 g/dL or > 2 g/dL below the lower limit of normal). This is caused by the physical crowding of normal blood-forming cells by malignant plasma cells in the bone marrow.
- B – Bone Lesions: The presence of one or more osteolytic (bone-destroying) lesions detected by skeletal survey, CT, or PET-CT.
Non-CRAB MDEs (MM Biomarkers of Malignancy)
In addition to CRAB, the 2014 MWG criteria established high-risk biomarker thresholds that, if met, also define the patient as having active MM, even without CRAB symptoms. Smoldering Multiple Myeloma (SMM) patients must also be absent of these:
- Bone Marrow Plasma Cells (BMPC): Clonal BMPC ≥ 60%.
- Free Light Chain Ratio (FLCr): Involved/Uninvolved FLCr ≥ 100.
- Focal Lesions on MRI/PET-CT: More than one focal bone lesion detected by MRI or PET-CT (even if less than 5 mm).
Non-Specific Symptoms and Immunoparesis
While Smoldering Multiple Myeloma (SMM) patients are defined as asymptomatic, some may report minor, vague complaints that can overlap with other conditions:
- Fatigue: Mild, non-debilitating fatigue is a common non-specific symptom. However, for the Smoldering Multiple Myeloma (SMM) diagnosis to remain, this fatigue cannot be conclusively attributed to anemia or any other MDE.
- Increased Infection Risk: Due to a process called immunoparesis (the suppression of normal immunoglobulin production), Smoldering Multiple Myeloma (SMM) patients often have low levels of healthy antibodies. While not a “symptom” itself, this can lead to an increased susceptibility to infection, which is a significant clinical consideration.
The clinical picture of Smoldering Multiple Myeloma (SMM) is one of silent biological progression, with the most important clinical finding being the potential for rapid change, necessitating the strategy of close active surveillance.
Investigations and Diagnosis of Smoldering Multiple Myeloma
The diagnosis of Smoldering Multiple Myeloma (SMM) is a comprehensive process guided by the criteria established by the International Myeloma Working Group (IMWG). The goal is to establish the presence of a high tumor burden while rigorously excluding any signs of end-organ damage.
IMWG Diagnostic Criteria for Smoldering Multiple Myeloma
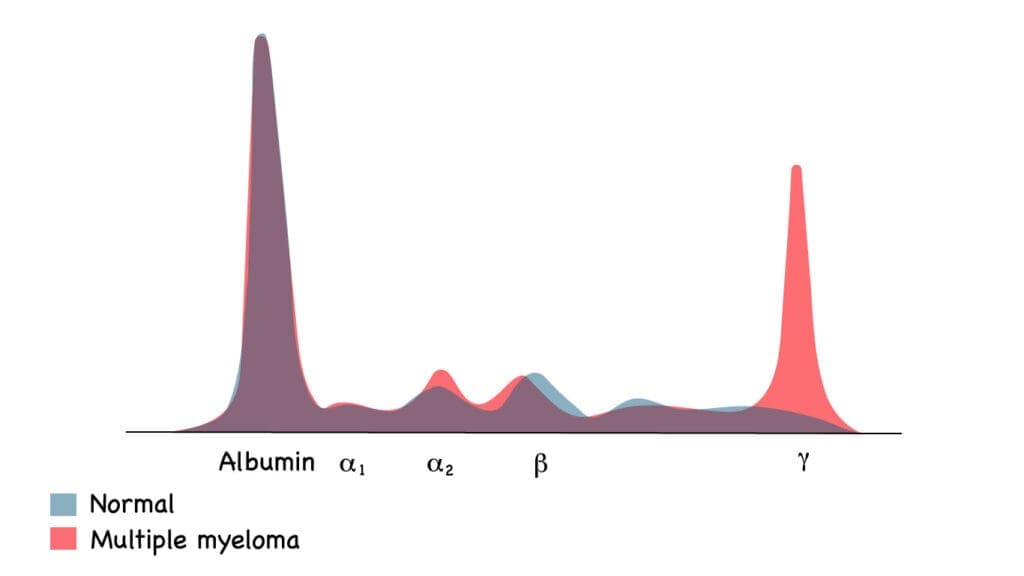
A diagnosis of Smoldering Multiple Myeloma (SMM) requires the simultaneous fulfillment of three distinct criteria:
- Serum Monoclonal Protein (M-protein): The level of IgG or IgA monoclonal protein in the serum must be ≥ 3 g/dL OR the urinary monoclonal protein (Bence-Jones protein) must be ≥ 500 mg per 24-hour collection.
- Clonal Bone Marrow Plasma Cells (BMPC): The proportion of clonal plasma cells in a bone marrow aspirate and biopsy must be between 10% and 60%. If the BMPC reaches ≥ 60%, the patient is immediately classified as having active Multiple Myeloma, even without CRAB symptoms.
- Absence of Myeloma-Defining Events (MDEs): The patient must demonstrate a complete absence of signs or symptoms of end-organ damage that can be attributed to the plasma cell disorder. This specifically excludes the CRAB criteria (Hypercalcemia, Renal failure, Anemia, Bone lesions) and the MM Biomarkers of Malignancy (e.g., BMPC ≥ 60%, FLCr ≥ 100, or > 1 focal bone lesion).
Essential Laboratory Studies
A set of specialized laboratory tests is mandatory to establish the tumor burden and rule out early organ damage.
| Study | Purpose | Key Findings in SMM |
| Complete Blood Count (CBC) | Screens for anemia. | Hemoglobin level is typically normal, as the bone marrow is not yet crowded enough to cause significant suppression. |
| Chemistry Panel | Checks for end-organ damage. | Serum Calcium and Creatinine levels must be normal to exclude the ‘C’ and ‘R’ of CRAB. |
| Serum Protein Electrophoresis (SPEP) & Immunofixation | Quantifies and identifies the M-protein. | SPEP shows a characteristic sharp spike (M-spike); Immunofixation confirms the immunoglobulin isotype (e.g., IgG Kappa). |
| Serum Free Light Chain (SFLC) Assay and Ratio | Measures the light chains not bound to heavy chains. | The ratio of the involved light chain to the uninvolved light chain (FLCr) is calculated. A ratio ≥ 100 automatically classifies the patient as active MM. |
| 24-hour Urine Protein Electrophoresis (UPEP) & Immunofixation | Quantifies and identifies M-protein in the urine. | Identifies Bence-Jones proteinuria (urinary free light chains), required to be < 500 mg/day for a standard SMM diagnosis. |
| Bone Marrow Aspirate and Biopsy | Directly assesses the percentage of clonal plasma cells. | Confirms 10%-60% infiltration. Also allows for Fluorescence In Situ Hybridization (FISH) testing to identify key genetic abnormalities (del(17p), t(4;14), etc.) for risk stratification. |
Imaging Studies (Exclusion of Bone Disease)
Since bone lesions are a hallmark of active MM, comprehensive imaging is required to exclude them. The sensitivity of imaging techniques has evolved, leading to changes in diagnostic standards.
- Low-Dose Computed Tomography (LDCT) of the Whole Body: This is the current standard for detecting bone lesions, as it is more sensitive than the traditional skeletal survey (X-rays).
- Positron Emission Tomography (PET)-CT or Magnetic Resonance Imaging (MRI) of the Spine and Pelvis: These highly sensitive methods are crucial for identifying early focal bone lesions that may not be apparent on CT or X-ray. The presence of more than one focal bone lesion detected by MRI or PET-CT (even if small and clinically asymptomatic) is considered an MM Biomarker of Malignancy and automatically reclassifies the patient from Smoldering Multiple Myeloma (SMM) to active, requiring treatment.
These investigations are essential not only for achieving the Smoldering Multiple Myeloma (SMM) diagnosis but also for gathering the key prognostic information (like the FLCr and genetic markers) needed to determine the patient’s individual risk of progression.
Risk Stratification and Prognosis in Smoldering Multiple Myeloma (SMM)
Risk stratification in Smoldering Multiple Myeloma (SMM) is the process of predicting which patients are most likely to progress to symptomatic Multiple Myeloma (MM) and require treatment. Because Smoldering Multiple Myeloma (SMM) is highly heterogeneous, distinguishing between low-risk patients (who can be safely monitored) and high-risk patients (who face imminent progression) is critical for management decisions.
Overall Progression Risk
Smoldering Multiple Myeloma (SMM) has an inherently higher risk of progression than Monoclonal Gammopathy of Undetermined Significance (MGUS), but this risk is not uniform over time.
- Average Annual Risk: The overall risk of progression to active MM is estimated to be approximately 10% per year for the first five years following diagnosis.
- Declining Risk: The risk significantly drops off after the initial period:
- Years 6-10: Approximately 3% per year.
- After Year 10: Approximately 1% per year (approaching the risk level of MGUS).
This pattern highlights that the patients most likely to progress rapidly are typically those who transition in the first few years, underscoring the necessity of accurate early risk stratification.
High-Risk SMM and the IMWG Criteria
The International Myeloma Working Group (IMWG) established widely adopted criteria to define High-Risk SMM patients who have a greater than 50% risk of progressing within two years.
This stratification often uses a combination of three key factors, sometimes referred to as the “20/2/20“ approach (though the IMWG uses slightly different thresholds for the M-protein).
| IMWG Risk Factor | Threshold for High Risk | Description |
| Bone Marrow Plasma Cell (BMPC) Percentage | > 20% | A high percentage of clonal plasma cells crowding the bone marrow. |
| Serum M-protein (SMP) Level | ≥ 3 g/dL | A large volume of monoclonal protein in the blood. |
| Involved/Uninvolved Free Light Chain Ratio (FLCr) | ≥ 20 | A significant imbalance between the malignant light chain and the healthy light chain. |
Risk Stratification by Number of Risk Factors
The number of these risk factors a patient possesses predicts their rate of progression:
- Low-Risk SMM: 0 risk factors.
- Intermediate-Risk SMM: 1 risk factor.
- High-Risk SMM: 2 or 3 risk factors. These patients have the fastest progression kinetics and are often the candidates for early intervention clinical trials.
The Importance of Cytogenetic Risk Factors
While the IMWG criteria focus on tumor burden and protein levels, genetic and cytogenetic testing provides crucial independent prognostic information. This testing is performed via FISH (Fluorescence In Situ Hybridization) on the plasma cells obtained from the bone marrow biopsy.
Specific high-risk genetic abnormalities are strongly associated with aggressive disease and rapid progression:
- Deletion of 17p (del(17p)): The loss of the short arm of chromosome 17, which contains the p53 tumor suppressor gene. This is one of the most critical high-risk markers, leading to impaired DNA repair and drug resistance.
- Translocations:
- t(4;14): Associated with high expression of FGFR3 and MMSET.
- t(14;16) and t(14;20): Less common but also confer poor prognosis.
The presence of any of these high-risk genetic lesions, regardless of the patient’s BMPC or M-protein levels, places them in a high-risk category, warranting aggressive surveillance or consideration for clinical trials.
Clinical Implications
The result of this stratification directly guides the management plan:
- Low- to Intermediate-Risk SMM: Management remains Active Surveillance (“Watch and Wait”). These patients are monitored closely for signs of progression (monthly or quarterly blood tests) but do not receive immediate treatment.
- High-Risk SMM: These patients are typically referred to specialized centers for enrollment in Clinical Trials that investigate early intervention strategies using drugs like lenalidomide, sometimes combined with monoclonal antibodies (e.g., daratumumab). The goal is to delay or prevent the onset of symptomatic MM.
Differential Diagnosis of Smoldering Multiple Myeloma
| Condition | Serum M-protein (M-spike) | Clonal Bone Marrow Plasma Cell (BMPC) % | Myeloma-Defining Events (MDEs) / CRAB Criteria | Key Differentiating Feature |
| Monoclonal Gammopathy of Undetermined Significance (MGUS) | < 3 g/dL | < 10% | Absent | Lowest risk of progression (approx 1% per year). |
| Smoldering Multiple Myeloma (SMM) | ≥ 3 g/dL | 10% – 60% | Absent | Intermediate to High risk of progression, necessitating close surveillance. |
| Active Multiple Myeloma (MM) | Variable (often high) | ≥ 10% | Present. Includes CRAB (Hypercalcemia, Renal failure, Anemia, Bone lesions) OR Biomarkers (e.g., BMPC, ≥ 60%, FLCr ≥ 100, > 1 focal lesion). | Requires immediate systemic treatment. |
| Waldenström Macroglobulinemia (WM) | Variable | Variable Lymphoplasmacytic Infiltration | Typically Absent (No CRAB) | M-protein is always IgM type. Associated with lymphadenopathy and hyperviscosity syndrome. |
| AL Amyloidosis (Primary) | Variable (often small or absent) | Variable Plasma Cell Infiltration | Absent (No CRAB) | Presence of amyloid deposits in vital organs (e.g., heart, kidneys) confirmed by biopsy. |
| Solitary Plasmacytoma | Variable | 0% BMPC (outside the lesion) | Absent | Only a single localized bone or soft-tissue tumor of plasma cells, no evidence of systemic disease. |
Clinical Management: Observation vs. Early Treatment
The management approach is determined almost entirely by the risk stratification, specifically whether the patient is classified as High-Risk or Low/Intermediate-Risk.
The core principle is that low-risk Smoldering Multiple Myeloma (SMM) is not treated with chemotherapy due to the side effects outweighing the benefits, whereas high-risk Smoldering Multiple Myeloma (SMM) is increasingly treated early to prevent progression.
The fundamental goal in managing Smoldering Multiple Myeloma (SMM) is to prevent or delay the progression to Active Multiple Myeloma (MM) while maintaining the patient’s quality of life.
Active Surveillance (“Watch and Wait”)
This is the standard of care for Low and Intermediate-Risk SMM patients.
Clinical trials have consistently shown that giving immediate treatment to all Smoldering Multiple Myeloma (SMM) patients does not improve overall survival compared to waiting until the disease progresses to active MM. Therefore, for lower-risk patients, the toxicities and side effects of continuous therapy are deemed more harmful than the disease itself.
Active surveillance involves rigorous, scheduled monitoring to catch the moment the disease progresses to active MM.
- Initial Period (First 1-2 years): Patients are typically monitored with blood and urine tests every 2 to 4 months.
- Stabilized Disease (After 2 years): If the disease remains stable, the monitoring frequency may be reduced to every 4 to 6 months.
- Annual Reassessment: A comprehensive reassessment, including updated whole-body CT or MRI, is often performed yearly or biannually to ensure no new Myeloma-Defining Events (MDEs) have silently developed.
Early Intervention
This approach is considered for High-Risk SMM patients, as defined by aggressive tumor burden markers (e.g., BMPC > 20%, FLCr > 20, or high-risk genetics).
High-risk Smoldering Multiple Myeloma (SMM) patients have an estimated progression rate of 50% – 80% within two years. Clinical trials (like GEM-CESAR trial) have demonstrated that early treatment in this specific group can significantly reduce the risk of progression and potentially lead to deeper responses compared to waiting.
Early intervention is typically administered through clinical trials and often involves combination therapy regimens similar to those used for newly diagnosed MM, but sometimes for a limited duration.
| Drug Class | Examples | Role in Early Intervention |
| Immunomodulatory Drugs (IMiDs) | Lenalidomide (Revlimid) | Used alone or in combination to inhibit plasma cell growth and modulate the immune system. |
| Proteasome Inhibitors (PIs) | Bortezomib (Velcade) | Drugs that disrupt protein homeostasis in the plasma cells, leading to cell death. |
| Monoclonal Antibodies (mAbs) | Daratumumab (Darzalex) | Targets the CD38 protein on the surface of plasma cells, leading to direct cell killing and enhanced immune response. Often used in combination with IMiDs. |
The goal of early intervention is to achieve a sustained deep response to “reset the clock” and prevent the progression to symptomatic MM, effectively converting a high-risk Smoldering Multiple Myeloma (SMM) patient back into a patient with a lower, more stable disease state.
Complications of Smoldering Multiple Myeloma
While Smoldering Multiple Myeloma (SMM) is technically defined by the absence of symptomatic complications, it’s crucial to recognize that the disease state itself leads to several subclinical and long-term risks.
These complications don’t always meet the full diagnostic criteria for Active Multiple Myeloma (MM), but they still significantly impact a patient’s health and quality of life.
Inherent Risk: Progression to Active Multiple Myeloma
The single greatest complication of Smoldering Multiple Myeloma (SMM) is its almost guaranteed eventual progression to active, symptomatic Multiple Myeloma. As discussed previously, the risk is highest in the initial years, averaging about 10% per year for the first five years. However, this risk is continuous and requires lifelong monitoring.
Once the disease crosses the threshold into Active MM (meeting CRAB criteria or the MM biomarkers like BMPC ≥ 60%), the patient faces the acute complications of the malignancy and requires immediate, intensive treatment, which comes with significant side effects and financial burdens.
Bone Health Complications (Subclinical Bone Damage)
While gross lytic lesions (the ‘B’ in CRAB) are absent in Smoldering Multiple Myeloma (SMM), the underlying biology of plasma cell activity begins to disrupt bone remodeling.
- Increased Bone Turnover: The malignant plasma cells secrete cytokines (like IL-6 and RANKL) that activate osteoclasts (cells that break down bone) and suppress osteoblasts (cells that build bone). This imbalance leads to localized bone loss.
- Osteopenia/Osteoporosis: Many SMM patients are found to have subclinical or pre-existing osteoporosis or osteopenia upon initial workup. The continuous background osteoclast activation accelerates this bone loss.
- Fracture Risk: The combination of underlying bone thinning and ongoing myeloma-related bone dysregulation increases the risk of pathologic fractures, even without a major lytic lesion being present.
Immunological Complications (Immunoparesis)
This is one of the most common and clinically relevant complications, resulting from the suppression of the patient’s normal immune function.
- Suppression of Normal Immunoglobulins: The proliferation of a single clone of plasma cells (producing the M-protein) often leads to the suppression of the healthy, polyclonal plasma cells that produce normal IgG, IgA, and IgM. This state is called immunoparesis.
- Increased Infection Risk: The low levels of functional, healthy antibodies leaves the patient vulnerable to infection. SMM patients have been shown to have a higher rate of serious bacterial infections, particularly encapsulated bacteria (e.g., S. pneumoniae, H. influenzae).
- Vaccine Response Impairment: Even if vaccinated, the patient’s ability to mount a robust, protective antibody response to new pathogens may be impaired due to the abnormal immune environment.
Related Monoclonal Gammopathy Complications
The presence of the abnormal monoclonal protein (M-protein) itself, even if not meeting CRAB criteria, can cause specific disorders.
- Peripheral Neuropathy: A small subset of Smoldering Multiple Myeloma (SMM) patients may develop peripheral neuropathy (numbness, tingling, pain in the hands or feet) that is directly attributed to the M-protein, either through direct toxicity or through deposition in the nerve sheaths.
- AL Amyloidosis or MGRS: While rare, the M-protein light chains produced in Smoldering Multiple Myeloma (SMM) can cause other serious conditions collectively known as M-protein-Related Kidney/Systemic disorders (MGRS), such as the development of AL amyloidosis or M-protein deposition disease. These require specific diagnostic workups and treatment distinct from MM.
Psychological and Monitoring Burden
While not a biological complication, the need for constant surveillance places a significant burden on the patient.
- Anxiety and Uncertainty: Living with an asymptomatic cancer precursor, where progression is a constant risk, often leads to significant anxiety, depression, and psychological distress (“watch and wait” anxiety).
- Monitoring Fatigue: The frequent medical appointments, blood draws, and scans required for Active Surveillance can be time-consuming, stressful, and impact the patient’s sense of normalcy.
Management of Associated Complications
Even without symptomatic MM, specific complications related to the plasma cell disorder must be managed.
Bone Health Management
While bone lesions define active MM, Smoldering Multiple Myeloma (SMM) patients have increased osteoclast activity (bone breakdown) which leads to bone loss and fracture risk.
- Bisphosphonates or Denosumab: These bone-strengthening agents are not routinely recommended for SMM unless the patient has a very high fracture risk or documented osteoporosis. Their use is typically reserved for active MM.
- Vitamin D and Calcium: Supplementation is highly recommended to maintain general bone health.
Infection Prevention
Due to immunoparesis (suppression of healthy IgG, IgA, or IgM production), Smoldering Multiple Myeloma (SMM) patients are at a slightly elevated risk of bacterial infection.
- Vaccinations: Patients should be kept up-to-date on routine vaccinations, particularly influenza, pneumococcal, and COVID-19 vaccines.
- Prophylactic IVIg: Intravenous Immunoglobulin (IVIg) replacement is generally reserved for patients with recurrent serious infections and very low normal immunoglobulin levels, a rare occurrence in SMM.
Frequently Asked Questions (FAQs)
What is the single most critical factor distinguishing SMM from MGUS?
The clonal burden and risk of progression. SMM has a BMPC percentage of 10%-59% or an M-protein ≥ 3 g/dL (or both), while MGUS must have BMPC <10% and M-protein <3 g/dL. The annual risk of progression to MM for SMM is significantly higher (~10% in the first 5 years) compared to MGUS (~ 1% per year).
Why is MRI mandatory in the workup of a suspected SMM patient?
MRI is the most sensitive imaging modality for detecting focal bone marrow lesions. The finding of more than one focal lesion (≥ 5 mm) on MRI reclassifies an asymptomatic patient from SMM to Active Multiple Myeloma (SLiM criteria), mandating immediate treatment.
Should a patient with high-risk SMM and t(4;14) be treated immediately?
While t(4;14) is a high-risk feature, treatment initiation must still be based on the IMWG criteria. Unless the patient meets a SLiM criterion (e.g., BMPC ≥ 60%, sFLCr ≥ 100$, or >1 focal lesion), or is enrolled in a clinical trial, the standard of care remains observation. However, the presence of t(4;14) significantly increases the progression risk, reinforcing the need for closer monitoring and consideration of trial enrollment.
Glossary of Key Medical Terms
- Clonal Plasma Cells: A population of plasma cells originating from a single abnormal precursor cell, producing a single type of monoclonal protein.
- CRAB Criteria: The traditional criteria for active Multiple Myeloma: Calcium elevation, Renal failure, Anemia, and Bone lesions.
- FISH: Fluorescence In Situ Hybridization. A cytogenetic technique used to detect specific chromosomal abnormalities (like t(4;14) or del(17p)) in plasma cells.
- Hyperdiploidy: The presence of more than the normal diploid number of chromosomes, specifically trisomies of odd-numbered chromosomes (3, 5, 7, etc.), a common genetic feature in plasma cell disorders.
- M-protein: Monoclonal protein (or paraprotein), an abnormal immunoglobulin or its components (light chains) produced by the clonal plasma cells.
- MGUS: Monoclonal Gammopathy of Undetermined Significance. The earliest, most benign precursor state of Multiple Myeloma.
- SLiM Criteria: Sixty, Light Chain Ratio, MRI. Biomarkers of Malignancy that reclassify SMM to Active MM (BMPC ≥ 60%, sFLCr ≥ 100, or >1 focal lesion on MRI).
- sFLCr: Serum Free Light Chain Ratio. The ratio of the involved free light chain (e.g., Kappa) to the uninvolved free light chain (e.g., Lambda).
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Rajkumar, S.V., Kumar, S., Lonial, S. et al. Smoldering multiple myeloma current treatment algorithms. Blood Cancer J. 12, 129 (2022). https://doi.org/10.1038/s41408-022-00719-0
- Rajkumar, S. V., Bergsagel, P. L., & Kumar, S. (2024). Smoldering Multiple Myeloma: Observation Versus Control Versus Cure. Hematology/oncology clinics of North America, 38(2), 293–303. https://doi.org/10.1016/j.hoc.2023.12.001
- Vaxman, I., & Gertz, M. A. (2022). How I approach smoldering multiple myeloma. Blood, 140(8), 828–838. https://doi.org/10.1182/blood.2021011670
- Lionetti, M., Da Vià, M. C., Albano, F., Neri, A., Bolli, N., & Musto, P. (2021). Genomics of Smoldering Multiple Myeloma: Time for Clinical Translation of Findings?. Cancers, 13(13), 3319. https://doi.org/10.3390/cancers13133319