TL;DR
Hereditary spherocytosis is an inherited hemolytic anemia due to a defect in a red cell membrane protein. It has mainly an autosomal dominant inheritance pattern.
- Signs and symptoms ▾:
- Mild to moderate anemia
- Intermittent jaundice precipitated by pregnancy, fatigue or infection
- Moderate splenomegaly
- Presence of pigment gallstones
- Aplastic crises triggered by an acute parvovirus B19 infection.
- Causes of hereditary spherocytosis ▾: Genetic mutations. Most common mutations are mutations found in the ankyrin, spectrin, band 3 or band protein 4.2.
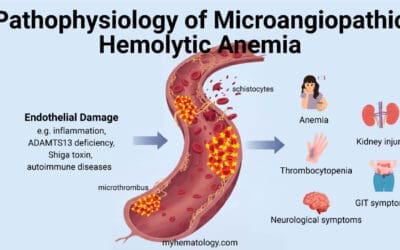
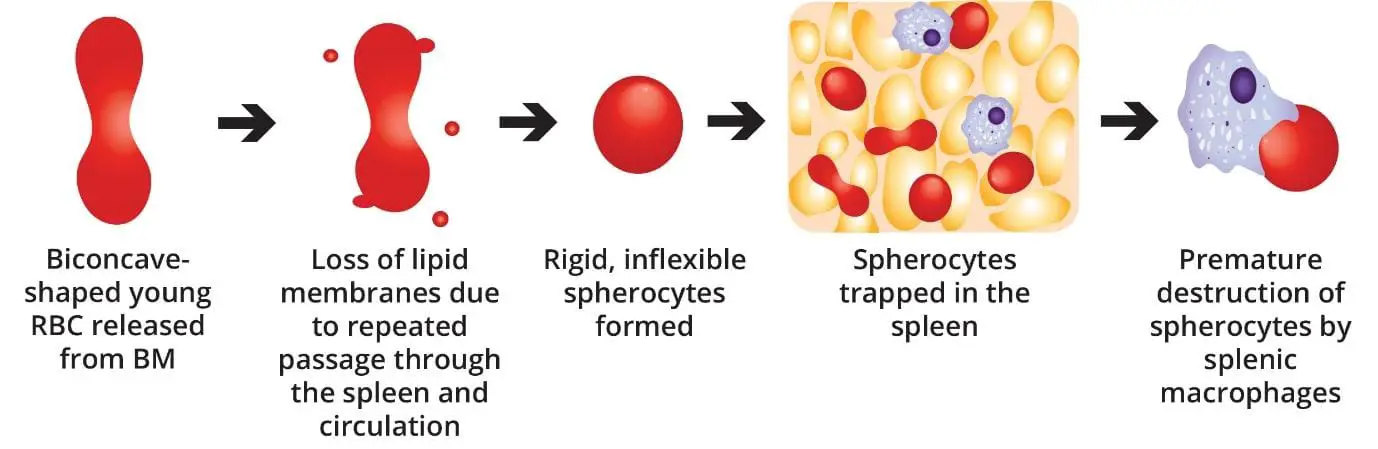
- Pathophysiology ▾: Normal-shaped young RBCs are produced by the bone marrow → repeated passage through the spleen and circulation leads to loss of membrane surface area releasing bilayer lipids in the form of cytoskeleton-free vesicles → spherocytes (rigid, inflexible and less deformable) → premature destruction of spherocytes by splenic macrophages (extravascular hemolysis).
- Laboratory Investigations ▾:
- Full blood count: variable hemoglobin level and reticulocytosis.
- Peripheral blood smear: Numerous polychromatic spherocytes.
- Bone marrow smear: Markedly hypercellular with erythroid hyperplasia
- ↑ serum bilirubin.
- ↑ urine urobilinogen.
- ↓ serum haptoglobin.
- Negative direct antiglobulin (Coombs) test.
- ↑ Osmotic fragility test
- ↓ fluorescent signal in flow cytometric analysis of eosin-5-maleimide binding.
- Positive family history
- Treatment and management of hereditary spherocytosis ▾: Folic acid supplement, splenectomy in severe cases and prophylactic penicillin V lifelong post splenectomy
*Click ▾ for more information
What is hereditary spherocytosis?
Hereditary spherocytosis is the most common hereditary (inherited) hemolytic anemia in northern Europeans. Hemolytic anemias are defined as those anemias that result from an increase in red cell destruction rate.
Hereditary hemolytic anemias are the result of ‘intrinsic’ red cell defects whereas acquired hemolytic anemias are usually the result of an extracorpuscular or environmental change. Paroxysmal nocturnal haemoglobinuria is the exception because although it is an acquired disorder, the red cells have an intrinsic effect.
What is hemolytic anemia?
Hemolytic anemia is a condition in which red blood cells are destroyed faster than the bone marrow can produce new ones. This premature destruction of red blood cells, known as hemolysis, leads to a decrease in their overall number, resulting in anemia.
Intravascular and extravascular hemolysis are two distinct processes of red blood cell (RBC) destruction. The key difference lies in where the breakdown occurs. Intravascular hemolysis happens within the blood vessels, while extravascular hemolysis occurs outside of them, primarily in the spleen and liver.
Comparison between Intravascular and Extravascular Hemolysis
| Feature | Intravascular Hemolysis | Extravascular Hemolysis |
| Location | Within blood vessels (e.g., arteries and veins) | Outside blood vessels, in the reticuloendothelial system (spleen, liver, and bone marrow) |
| Mechanism | RBCs are directly lysed or fragmented in the circulation, often due to physical trauma, toxins, or complement system activation. | Macrophages in the spleen and liver engulf and destroy damaged or antibody-coated RBCs. |
| Causes | Microangiopathic hemolytic anemia (MAHA), mechanical heart valves, severe infections (e.g., malaria), drug-induced immune hemolysis, and some autoimmune conditions. | Hereditary spherocytosis, sickle cell disease, warm autoimmune hemolytic anemia, and some drug-induced anemias. |
| Pathophysiology | Hemoglobin is released directly into the plasma. This free hemoglobin is bound by haptoglobin, which is then cleared from the circulation. When haptoglobin is overwhelmed, free hemoglobin is filtered by the kidneys. | Hemoglobin is processed within the macrophages, where it’s broken down into its components, including unconjugated bilirubin. |
Causes of Hemolytic Anemia
Hemolytic anemia can be either inherited or acquired.
Inherited Causes (Intrinsic Defects)
These are genetic conditions that affect the red blood cells themselves, making them fragile and prone to destruction.
- Membrane Defects: Such as hereditary spherocytosis, which causes red blood cells to be spherical and less flexible.
- Enzyme Deficiencies: Like G6PD deficiency, where a lack of a key enzyme makes cells susceptible to oxidative stress.
- Hemoglobinopathies: Disorders like sickle cell anemia and thalassemia, which result in abnormal hemoglobin that can distort the red blood cell shape.
Acquired Causes (Extrinsic Factors)
These are external factors that cause the destruction of otherwise normal red blood cells.
- Autoimmune Conditions: The immune system mistakenly produces antibodies that attack and destroy red blood cells, as seen in autoimmune hemolytic anemia.
- Infections: Certain infections, like malaria, can directly destroy red blood cells.
- Mechanical Damage: The physical trauma of passing through a damaged heart valve or a narrowed blood vessel can fragment red blood cells.
- Toxins or Drugs: Exposure to certain toxins, snake venoms, or medications can trigger hemolysis.
What causes hereditary spherocytosis?
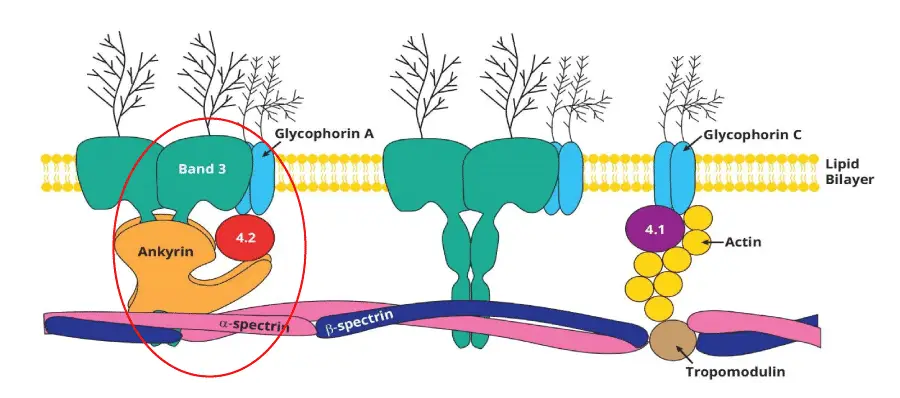
Hereditary spherocytosis is usually caused by defects in the membrane proteins involved in the vertical interactions between the membrane skeleton and the lipid bilayer of the red cell.
The most common mutations affect proteins include:
- Ankyrin (ANK1)
- Spectrin (SPTA1, SPTB)
- Protein 4.2
- Band 3 protein (SLC4A1)
These proteins form a network that links the inner cytoskeleton to the outer lipid bilayer of the cell membrane. Mutations in these genes lead to a quantitative or qualitative defect in these proteins, weakening the structural integrity of the RBC.
The inheritance pattern of this disorder is autosomal dominant. Only 1 mutant allele needs to be inherited for the patient to be symptomatic.
Pathophysiology of hereditary spherocytosis
As the RBC travels at a very high speed in the vascular lumen, the RBC inadvertently knocks against the vascular lumen wall and also other cells. The weakened membrane loses its ability to maintain its shape.
As the cell circulates and passes through the narrow capillaries, particularly in the spleen, it sheds small, lipid-rich fragments from its membrane. The loss of membrane may be caused by the release of parts of the lipid bilayer that are not supported by the skeleton. The marrow produces red cells that are normal biconcave shapes but this repeated loss of membrane surface area, without a corresponding loss of cell volume, causes the once biconcave, disc-shaped cell to become a smaller, more rigid, and less flexible spherocyte. Ultimately, yhe spherocyte’s reduced surface-area-to-volume ratio makes it fragile and unable to deform to pass through the tight spaces of the splenic microcirculation where they are prematurely phagocytosed and destroyed by the splenic macrophages.
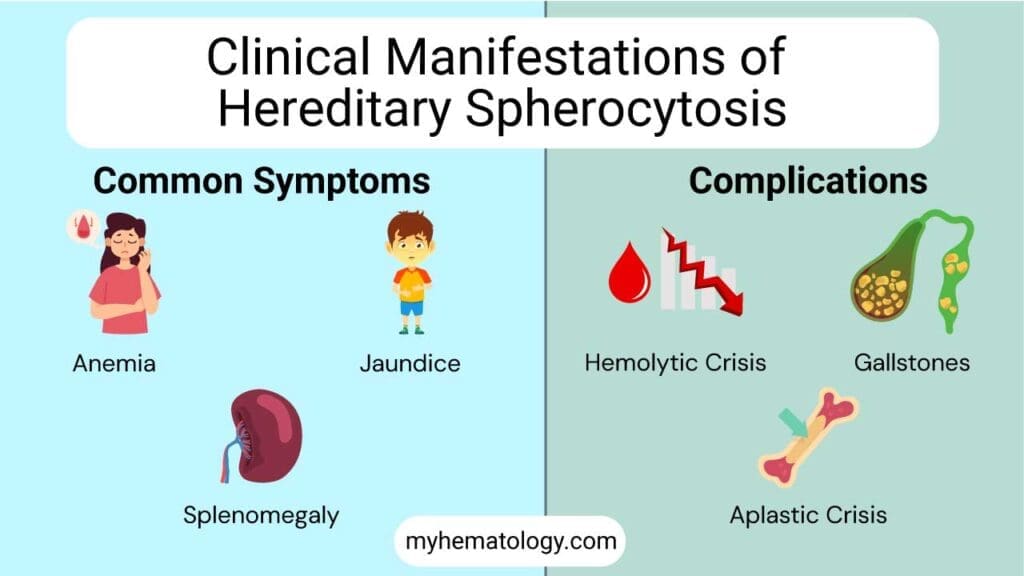
Signs and Symptoms of Hereditary Spherocytosis
Hereditary spherocytosis (HS) is a genetic condition that causes red blood cells to be abnormally spherical and fragile, leading to chronic hemolytic anemia. The signs and symptoms vary widely depending on the severity of the disease, which is classified as mild, moderate, or severe.
Common Signs and Symptoms
- Anemia: A shortage of red blood cells is the core problem. This can manifest as fatigue, weakness, pale skin, and shortness of breath due to the body’s reduced ability to transport oxygen.
- Jaundice: The premature destruction of red blood cells releases a yellow pigment called bilirubin. When the liver is overwhelmed by this excess bilirubin, it builds up in the body, causing a yellowing of the skin and the whites of the eyes.
- Splenomegaly: The spleen is the primary site where these fragile, spherical red blood cells are trapped and destroyed. Over time, the constant workload causes the spleen to enlarge, a condition called splenomegaly. This can sometimes be felt as a lump or cause discomfort in the abdomen.
Complications and Crises
Individuals with hereditary spherocytosis are prone to several complications, especially during periods of increased stress on the body, such as infections.
- Hemolytic Crisis: This occurs when there is a sudden and rapid increase in the rate of red blood cell destruction. It can be triggered by infections and results in a swift drop in hemoglobin levels, increased jaundice, and worsening splenomegaly.
- Gallstones: The high levels of bilirubin from ongoing red blood cell breakdown can form hard deposits in the gallbladder, leading to the formation of pigment gallstones. These can cause severe abdominal pain.
- Aplastic Crisis: This is a temporary but serious complication where the bone marrow abruptly stops producing new red blood cells. It’s often triggered by a viral infection, particularly parvovirus B19. An aplastic crisis leads to a sudden and severe worsening of anemia, which may require a blood transfusion.
How do I test for hereditary spherocytosis?
The diagnosis of hereditary spherocytosis (HS) is a multifaceted process that integrates a patient’s clinical history and physical exam findings with a series of targeted laboratory tests. The diagnostic workup typically proceeds in a step-wise fashion, starting with broad screening for hemolysis and narrowing down to specific tests for hereditary spherocytosis.
Initial Laboratory Investigations
The first step is to confirm the presence of hemolysis and rule out other common causes.
- Direct Antiglobulin Test (DAT) / Direct Coombs Test: This test is essential to differentiate hereditary spherocytosis from autoimmune hemolytic anemia, which can also cause spherocytes. A negative DAT rules out an autoimmune cause, strongly suggesting an intrinsic red blood cell defect like HS.
- Complete Blood Count (CBC) with Reticulocyte Count: A CBC often shows anemia. A key finding is an elevated mean corpuscular hemoglobin concentration (MCHC), which is an unusually high concentration of hemoglobin inside the red blood cells, a classic sign of spherocytes. The reticulocyte count will be high, reflecting the bone marrow’s attempt to compensate for the rapid destruction of red blood cells.
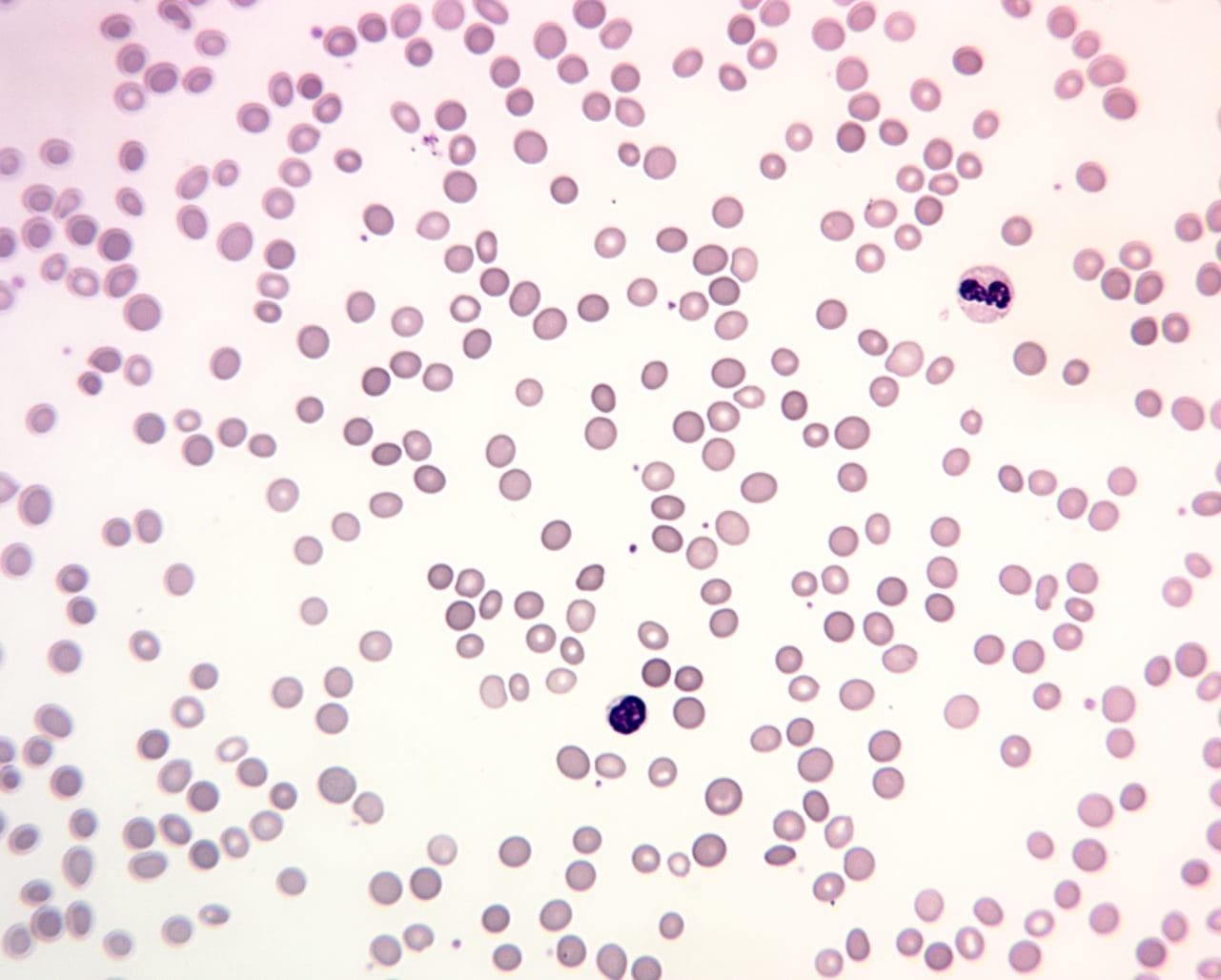
- Peripheral Blood Smear: The smear will reveal the characteristic presence of spherocytes, which are small, dense, spherical red blood cells that lack the central pallor of normal biconcave red blood cells. Other features of hemolysis, such as polychromasia (indicating an increase in reticulocytes), may also be present.
- Elevated unconjugated bilirubin: The breakdown of hemoglobin from destroyed RBCs leads to an increase in this pigment.
- Elevated Lactate Dehydrogenase (LDH): This enzyme is released from the inside of lysed red blood cells.
- Decreased haptoglobin: Haptoglobin binds to free hemoglobin and is quickly cleared from the blood, so its levels drop.
Specific Confirmatory Tests
If initial findings point toward an intrinsic red blood cell defect, specific tests are used to confirm the diagnosis of hereditary spherocytosis.
- Osmotic Fragility Test (OFT): This is a historical “gold standard” but is now being replaced by more sensitive methods. The test measures the susceptibility of red blood cells to lysis when exposed to a hypotonic saline solution. Spherocytes, with their reduced surface area, are more fragile and will lyse at a higher saline concentration than normal RBCs. An incubated OFT, performed after a period of incubation, is more sensitive for detecting milder cases.
- Eosin-5′-Maleimide (EMA) Binding Test: This is the most widely recommended and sensitive modern test for hereditary spherocytosis. It uses a fluorescent dye (EMA) that binds to the red blood cell membrane protein Band 3. In hereditary spherocytosis, a deficiency or defect in membrane proteins leads to reduced binding of the dye, which is measured by flow cytometry. A significant decrease in fluorescence intensity compared to a control sample is highly indicative of hereditary spherocytosis. This test is particularly useful for diagnosing mild or atypical cases that may be missed by the osmotic fragility test.
- Acidified Glycerol Lysis Test (AGLT): Similar to the osmotic fragility test, this test measures the time it takes for red blood cells to lyse in a buffered glycerol solution. Spherocytes have a shorter lysis time due to their increased fragility.
Advanced and Molecular Diagnostics
For complex or ambiguous cases, especially when the more common tests are inconclusive, more advanced investigations can be considered.
Ektacytometry: This highly specialized test directly measures the deformability of red blood cells under controlled stress. The cells of HS patients show decreased deformability and a characteristic abnormal curve, which can help differentiate hereditary spherocytosis from other red blood cell membrane disorders.
Genetic Testing: Since hereditary spherocytosis is a genetic disorder, identifying the specific mutation in genes like ANK1, SPTA1, or SPTB can provide a definitive diagnosis. This is not a routine first-line test due to its cost and complexity, but it can be valuable for confirming the diagnosis, especially in cases with an unclear family history or when other tests are equivocal.
How is hereditary spherocytosis treated or managed?
The treatment and management of hereditary spherocytosis (HS) are tailored to the severity of the disease, which can range from asymptomatic to severe and transfusion-dependent. The goal is not to cure the underlying genetic defect but to manage symptoms and prevent complications.
Supportive Care and Monitoring
For most patients with mild or moderate hereditary spherocytosis, the primary approach is supportive care and regular monitoring.
- Folic Acid Supplementation: Because the body is constantly working to produce new red blood cells to replace the ones being destroyed, there is a high demand for folate (vitamin B9), which is crucial for red blood cell production. Daily folic acid supplementation is recommended to prevent a megaloblastic crisis, a form of anemia caused by folate deficiency.
- Regular Monitoring: Patients should have regular check-ups to monitor for signs of worsening anemia, splenomegaly, or the development of gallstones. Abdominal ultrasounds can be used to check for the presence of gallstones, which can develop due to the chronic hemolysis.
- Phototherapy and Blood Transfusions: In newborns with HS, severe jaundice may be treated with phototherapy to break down bilirubin. In cases of severe anemia, especially during a crisis (e.g., an aplastic crisis caused by parvovirus B19 infection), blood transfusions may be necessary to quickly restore red blood cell levels.
Splenectomy
Splenectomy, the surgical removal of the spleen, is the most effective treatment for the chronic hemolysis associated with hereditary spherocytosis. It is considered the definitive treatment for severe hereditary spherocytosis and is a consideration for patients with moderate hereditary spherocytosis who have significant symptoms or complications. It is generally not performed on children under 6 years of age due to the increased risk of severe, life-threatening bacterial infections (e.g., from encapsulated bacteria like Streptococcus pneumoniae).
While highly effective, splenectomy has risks. The most serious is the lifelong increased susceptibility to overwhelming post-splenectomy infection (OPSI). To mitigate this risk, patients must receive specific vaccinations (e.g., against pneumococcus, meningococcus, and Haemophilus influenzae) before the surgery and may be placed on long-term prophylactic antibiotics. There is also a small risk of developing blood clots.
Management of Complications
Beyond the core treatment, managing complications is also a key part of care.
- Iron Overload: For patients who require repeated blood transfusions, there is a risk of iron overload. Iron chelation therapy may be used to remove excess iron from the body and prevent damage to organs.
- Gallstones: If symptomatic gallstones develop, a cholecystectomy (gallbladder removal) may be performed, often at the same time as a splenectomy if indicated.
Frequently Asked Questions (FAQs)
What is the most common defect in hereditary spherocytosis?
The most common defect in hereditary spherocytosis is a deficiency in the spectrin protein. Spectrin is a crucial component of the red blood cell membrane, providing structural support and maintaining its normal biconcave shape. When spectrin is deficient or abnormal, the red blood cells become sphere-shaped, which makes them more susceptible to destruction by the spleen.
What four main complications can occur in patients with hereditary spherocytosis?
- Hemolytic anemia: This is the most common complication, resulting from the premature destruction of red blood cells by the spleen. Symptoms include fatigue, weakness, shortness of breath, and pallor.
- Splenomegaly: The spleen becomes enlarged due to its increased workload in destroying damaged red blood cells. This can cause abdominal discomfort, fullness, and sometimes pain.
- Gallstones (Cholelithiasis): The breakdown of red blood cells releases bilirubin, which can accumulate in the gallbladder and form stones. This can lead to abdominal pain, nausea, and vomiting.
- Aplastic crisis: In rare cases, patients with hereditary spherocytosis may experience a sudden decrease in red blood cell production, resulting in a severe form of anemia. This can require blood transfusions or other interventions.
What is the life expectancy of a person with hereditary spherocytosis?
The life expectancy of a person with hereditary spherocytosis can vary widely depending on the severity of the condition and the effectiveness of treatment.
In mild cases, individuals may experience few symptoms and have a normal life expectancy. However, in more severe cases, frequent blood transfusions or splenectomy (removal of the spleen) may be necessary, which can improve quality of life and extend survival.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Anemia: Diagnosis and Treatment (Willis, 2016).
- Management of Anemia: A Comprehensive Guide for Clinicians (Provenzano et al., 2018)
- Goldberg S, Hoffman J. Clinical Hematology Made Ridiculously Simple, 1st Edition: An Incredibly Easy Way to Learn for Medical, Nursing, PA Students, and General Practitioners (MedMaster Medical Books). 2021.
- Zamora EA, Schaefer CA. Hereditary Spherocytosis. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539797/
- Turpaev, K., Bovt, E., Shakhidzhanov, S., Sinauridze, E., Smetanina, N., Koleva, L., Kushnir, N., Suvorova, A., & Ataullakhanov, F. (2025). An overview of hereditary spherocytosis and the curative effects of splenectomy. Frontiers in physiology, 16, 1497588. https://doi.org/10.3389/fphys.2025.1497588
- Wu, Y., Liao, L., & Lin, F. (2021). The diagnostic protocol for hereditary spherocytosis-2021 update. Journal of clinical laboratory analysis, 35(12), e24034. https://doi.org/10.1002/jcla.24034
- Achenjang, N.-S., Jadczak, E., Ryan, R. M., & Nock, M. L. (2025). Hereditary Spherocytosis: Review of Presentation at Birth. Children, 12(9), 1207. https://doi.org/10.3390/children12091207
- Tole, S., Dhir, P., Pugi, J., Drury, L. J., Butchart, S., Fantauzzi, M., Langer, J. C., Baker, J. M., Blanchette, V. S., Kirby-Allen, M., & Carcao, M. D. (2020). Genotype-phenotype correlation in children with hereditary spherocytosis. British journal of haematology, 191(3), 486–496. https://doi.org/10.1111/bjh.16750
- Polizzi, A., Dicembre, L. P., Failla, C., Matola, T. D., Moretti, M., Ranieri, S. C., Papa, F., Cenci, A. M., & Buttarello, M. (2025). Overview on Hereditary Spherocytosis Diagnosis. International journal of laboratory hematology, 47(1), 18–25. https://doi.org/10.1111/ijlh.14376