Procedure At A Glance
The purpose serologic cross-matching (blood compatibility test) is to detect ABO incompatibility and clinically significant antibodies in recipient serum/plasma against donor red blood cells.
Immediate Spin (IS) Phase
- Add 2 drops of Recipient Serum/Plasma and 1 drop of Donor Red Blood Cell Suspension (2-5%) into a test tube.
- Gently mix the tube and centrifuge immediately
- Gently dislodge the cell button and observe for agglutination (clumping).
- Interpretation: Detects ABO incompatibility. Any agglutination at this stage generally indicates an incompatibility.
37°C Incubation Phase
- If no agglutination in the IS phase, incubate the tube at 37°C for 15-30 minutes (or per validated protocol).
- After incubation, wash the red blood cells 3-4 times with normal saline.
Anti-Human Globulin (AHG) Phase
- Add 2 drops of Anti-Human Globulin (AHG) Reagent to the washed cell button.
- Gently mix the tube and centrifuge (e.g., 15-20 seconds at 3400 rpm).
- Gently dislodge the cell button and observe for agglutination.
- Interpretation: Detects clinically significant IgG antibodies (e.g., those reacting at body temperature). Agglutination indicates incompatibility.
Check Cells (Coombs Control Cells)
- If no agglutination is observed in the AHG phase, add 1 drop of IgG-sensitized control cells (check cells).
- Gently mix and centrifuge.
- Observe for agglutination.
- Interpretation: Agglutination MUST occur with check cells. If no agglutination, the AHG reagent was inactive or washing was inadequate (indicating a false negative result), and the test must be repeated. If agglutination occurs, the negative AHG result is valid.
- No Agglutination at Any Phase & Positive Check Cells: Donor unit is compatible for transfusion.
- Agglutination at Any Phase: Donor unit is incompatible and should NOT be transfused. Further investigation is required.
Introduction
A serological cross-matching, also known as a blood compatibility test, is a vital procedure performed before a blood transfusion to ensure compatibility between the donor’s red blood cells and the recipient’s serum or plasma.
In the realm of blood transfusions, ensuring blood compatibility between recipient and donor is paramount. This blood compatibility check or cross-matching minimizes the risk of potentially life-threatening hemolytic reactions, where the recipient’s immune system attacks the transfused red blood cells.
Here’s how cross-matching achieves this:
- Detects ABO incompatibility: Even a slight mismatch in the ABO blood group system can trigger immediate agglutination (clumping) and destruction of donor red blood cells upon transfusion. Cross-matching (blood compatibility test) directly checks for this incompatibility, ensuring only ABO-compatible blood is used.
- Identifies irregular antibodies: Some individuals develop antibodies against antigens on red blood cells other than the ABO system. These “irregular antibodies” can target donor red blood cells, leading to delayed hemolysis after transfusion. Cross- matching (blood compatibility test) specifically tests for the presence of such antibodies and their reaction with potential donor blood.
- Provides a final safeguard: While blood typing and antibody screening offer initial blood compatibility checks, cross-matching (blood compatibility test) acts as the final confirmation step. It directly exposes donor red blood cells to the recipient’s plasma/serum, simulating an actual transfusion and revealing any hidden incompatibilities.
Major vs. Minor Cross-matching
Both major and minor cross-matching (blood compatibility test) are performed in serologic compatibility testing for blood transfusions, but they serve different purposes and target different potential risks.
| Feature | Major Cross-matching | Minor Cross-matching |
| Function | Detects ABO incompatibility, strong irregular antibodies, and confirms overall compatibility. | Detects potential harm from donor antibodies to recipient red blood cells. |
| Test Components | Recipient serum/plasma with donor red blood cells. | Donor serum/plasma with recipient red blood cells. |
| Frequency | Mandatory for almost all transfusions. | Less frequent, done based on specific scenarios and institutional policies. |
| Sensitivity | Less sensitive. | More sensitive due to the AHG phase. |
| Antibody Detection | Readily reacting antibodies. | Weaker antibodies that might not be visible in the immediate spin phase. |
| Clinical Significance | Crucial for preventing immediate and delayed hemolytic reactions. | Additional safety measure, not always mandatory but important in specific cases. |
Approaches to Cross-matching (Blood Compatibility Test)
1. Group, Screen and Hold (GSH)
- Employed when no history of irregular antibodies exists and initial antibody screening (screen) yields negative results.
- Involves determining recipient ABO and RhD (D positive/negative) type.
- A compatible unit based on ABO and RhD is selected and “held” pending final compatibility testing.
- If the immediate cross-matching screen remains negative at a designated time point, the “held” unit is released for transfusion.
- Applicable to situations where blood transfusions are considered unlikely.
2. Group, Screen and Crossmatch (GXM)
- Mandatory when
- Recipient has a history of clinically significant antibodies.
- Current antibody screen detects unexpected antibodies.
- Recipient requires rare blood components.
- Follows ABO and RhD typing of recipient.
- Antibody screen is performed to detect irregular antibodies.
- If screen is negative, a full cross-matching (blood compatibility test) is conducted using patient plasma/serum and compatible donor RBCs. This involves:
- Immediate-spin phase: Detects ABO incompatibility and certain irregular antibodies.
- AHG phase: Enhances detection of weaker antibodies not evident in the immediate-spin phase.
- Only units demonstrably compatible in both phases are released for transfusion.
Principle of Serologic Cross-matching
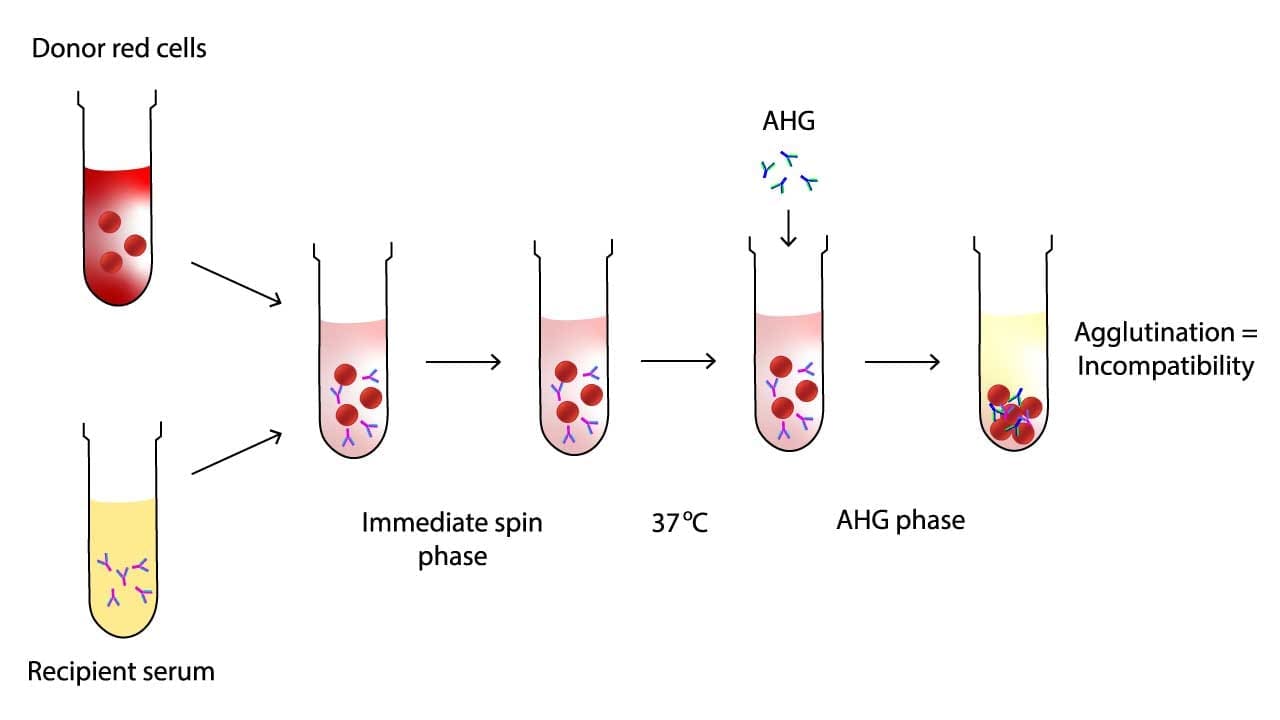
Serologic cross-matching (blood compatibility test) lies at the heart of ensuring safe blood transfusions. It works on two key principles: agglutination and antigen-antibody reactions.
Agglutination
Red blood cells (RBCs) have antigens on their surface, like unique molecular markers. If an incompatible antibody encounters its corresponding antigen, it binds to the RBC, causing agglutination (clumping). This clumping signifies incompatibility and potential for a destructive immune reaction if transfused.
Antigen-Antibody Reactions
The cross-matching (blood compatibility test) mixes recipient serum/plasma (containing antibodies) with donor RBCs (possessing antigens).
- If compatible, no reaction occurs.
- If incompatible, antibodies bind to their specific antigens on donor RBCs, triggering agglutination.
Major Cross-matching Steps
- Immediate-spin phase: Detects ABO incompatibility and strong irregular antibodies causing immediate agglutination.
- AHG (anti-human globulin) phase: Enhances detection of weaker antibodies by coating RBCs with antiglobulin, causing visible agglutination if antibodies are present.
Blood Compatibility Confirmation
Only units demonstrating no agglutination in both phases are deemed compatible and safe for transfusion.
Materials
- Glass tubes labeled for immediate spin phase
- Micropipettes and sterile tips
- 2% – 5% suspension of donor red blood cells (RBCs) in saline or EDTA solution
- Recipient serum/plasma
- Centrifuge
- Normal saline (0.9% NaCl) / Low ionic strength solution (LISS)
- Anti-human globulin (AHG) solution
- Coombs control cells (CCC)
Protocol
Immediate Spin Phase
The immediate spin phase is the first step in serologic cross-matching (blood compatibility test), aiming to detect ABO incompatibility and strong, room-temperature reacting irregular antibodies. It’s a quick and preliminary check before the more sensitive AHG phase.
Disclaimer: Protocols of serologic cross-matching (blood compatibility test) vary depending on regional standards, institutional policies, and specific reagents used due to the specific nature of such procedures and the need for strict adherence to established guidelines and regulations.
Reliable Resources:
- American Association of Blood Banks (AABB): https://www.aabb.org/
- International Society of Blood Transfusion (ISBT): https://www.isbtweb.org/
- College of American Pathologists (CAP): https://www.cap.org/
- Educational blood typing and cross-matching kits from reputable scientific supply companies.
Please consult these resources and adapt the protocol according to your specific requirements and institutional guidelines.
- Add 1 drop of diluted donor RBCs to the glass tube.
- Add 2 drops of recipient serum/plasma to the same tube.
- Briefly agitate the tube to ensure thorough mixing and incubate for 5 minutes at room temperature.
- Spin the tube at the specified speed and time according to your institutional protocol (typically 900 x g for 20 seconds).
- Observe for agglutination by tilting the tube slightly and examine under a good light source or with a mirror.
- Document the presence or absence of agglutination immediately.
Interpretation
- Agglutination: A positive reaction signifies potential incompatibility requiring further investigation in the AHG phase and possibly additional testing.
- No agglutination: A negative reaction suggests provisional blooc compatibility, but the cross-matching remains incomplete until the AHG phase confirms the absence of weaker antibodies.
Do not proceed with transfusion based solely on the immediate spin phase. A negative reaction in this phase requires confirmation through the 37°C and AHG phase.

AHG Phase
Some antibodies react weakly and might not cause visible clumping (agglutination) in the immediate spin phase. The AHG phase amplifies the reaction, making these weaker antibodies detectable, thereby preventing potentially harmful transfusion reactions.
- Increased sensitivity: This phase significantly enhances the sensitivity of the cross-matching (blood compatibility test) process, ensuring compatibility and minimizing transfusion risks.
- Safeguards patient well-being: By uncovering even weak incompatibilities, the AHG phase plays a crucial role in protecting patients from transfusion-related complications like hemolytic reactions, which can be severe and even life-threatening.
- Add 1 drop of LISS reagent to the previous immediate spin phase tubes with negative reactions.
- Briefly agitate the tube to ensure thorough mixing and incubate for 10 – 15 minutes at 37°C.
- Spin the tube at the specified speed and time according to your institutional protocol (typically 900 x g for 20 seconds).
- Observe for agglutination by tilting the tube slightly and examine under a good light source or with a mirror.
- Document the presence or absence of agglutination immediately for the 37°C phase.
- Wash the red cells with normal saline 3 times, discard the supernatant completely after the final wash other than the red cell button.
- Add 1 drop of AHG onto the red cells.
- Tilt the tube and gently swirl it to mix the AHG with the red cells, ensuring they are completely resuspended.
- Place the tube in the centrifuge and spin for 15-30 seconds at 900-1000 g or as per manufacturer’s instructions.
- Gently resuspend the cell buttons and observe for agglutination macroscopically.
- Grade and record agglutination if present.
- In the absence of agglutination after initial centrifugation, add Coombs control cells (CCC) and centrifuge as per manufacturer instructions. Observe and record any agglutination with CCC, confirming test validity.
Interpretation
- Compatible recipient and donor blood: This outcome indicates no agglutination (clumping) or hemolysis of red blood cells in any of the phases i.e. immediate spin, 37°C and AHG phase with agglutination only in CCC phase; suggesting compatible blood types and a safe transfusion.
- Incompatible recipient and donor blood: This indicates agglutination in any of the phases i.e. immediate spin, 37°C and AHG phase; usually due to ABO incompatibility or strong irregular antibodies, and signifies an unsafe transfusion risk.
- Inconclusive: In some cases, results might be unclear or require further investigation using additional tests or techniques before a definitive interpretation can be made.
Troubleshooting
When troubleshooting a serological cross-matching (blood compatibility test) protocol, identifying the root cause of unexpected results is important.
False Positives (Agglutination without true incompatibility)
False positives occur when there’s an apparent incompatibility (agglutination) that isn’t due to clinically significant donor red cell-recipient antibody reactions.
Common Causes
Improper Sample Handling/Contamination
- Fibrin Clots: Inadequately clotted or re-centrifuged samples can leave fibrin strands that mimic agglutination.
- Bacterial Contamination: Contamination in reagents or samples can cause non-specific agglutination.
- Contaminated Saline: Saline with particulate matter can lead to false readings.
Autoagglutination
- Cold Autoantibodies: Clinically insignificant cold autoantibodies (e.g., anti-I, anti-i) reactive at room temperature or colder can cause agglutination in the immediate spin phase. These often react with all cells, including the autocontrol.
- Rouleaux Formation: This “stack of coins” appearance of red blood cells, common in patients with abnormal plasma proteins (e.g., multiple myeloma, hypergammaglobulinemia), can be mistaken for true agglutination. It disperses with saline addition.
Non-specific Agglutination due to Reagents
- Dirty Glassware/Tubes: Residue can cause non-specific reactions.
- Over-centrifugation: Excessive centrifugation can pack cells so tightly they appear agglutinated.
- Contaminated/Expired Reagents: Anti-human globulin (AHG) or red cell suspensions that are contaminated or expired can lead to erroneous results.
Positive DAT (Direct Antiglobulin Test) on Recipient Cells
If the recipient’s red blood cells are already coated with antibody or complement in vivo, this can cause a positive AHG phase reaction regardless of donor cells, especially if the autocontrol is not run or misinterpreted.
Solution
- Check Sample Quality: Visually inspect serum/plasma for clots or turbidity. If fibrin is suspected, re-centrifuge the sample.
- Run an Autocontrol: Always include an autocontrol (recipient serum/plasma + recipient red cells). If the autocontrol is positive, it suggests autoagglutination or a positive DAT, not necessarily an incompatibility with donor cells.
- Perform Saline Replacement (for Rouleaux): Add a drop of saline to the apparent agglutinate and gently mix. Rouleaux will disperse, while true agglutination will remain.
- Warm the Sample (for Cold Autoantibodies): If cold autoantibodies are suspected (positive in immediate spin, negative at 37°C, and positive AHG), repeat the test using pre-warmed samples and reagents. Cold autoantibodies usually react strongest at immediate spin and often weaken or disappear at 37°C incubation.
- Wash Cells Thoroughly: Ensure adequate washing (at least 3-4 times) during the AHG phase to remove unbound globulins, which can neutralize the AHG reagent.
- Inspect Reagents and Equipment: Check expiry dates of reagents, ensure they are stored correctly, and use clean, dry tubes/glassware. Calibrate centrifuges regularly.
- Review Patient History: Look for conditions known to cause rouleaux or autoantibodies.
False Negatives (No agglutination despite true incompatibility)
False negatives are more dangerous as they suggest compatibility when a clinically significant incompatibility actually exists, potentially leading to a hemolytic transfusion reaction.
Common Causes
Failure to Add Reagents
- Missing Serum/Plasma: Most common cause – failure to add the recipient’s serum/plasma.
- Missing Donor Cells: Failure to add donor red cell suspension.
- Missing AHG Reagent: Critically, not adding AHG reagent will result in failure to detect IgG antibodies.
Inadequate Washing
- Insufficient Washing (AHG Phase): Residual unbound patient globulins (antibodies or complement) can neutralize the AHG reagent, preventing it from binding to sensitized red cells, leading to a false negative.
Inactive/Contaminated Reagents
- Weak/Inactive AHG Reagent: AHG that is expired, improperly stored, or contaminated can lose its reactivity.
- Weak Red Cell Suspensions: Too dilute a red cell suspension can make agglutination difficult to detect.
Improper Cell/Serum Ratio
- Prozone/Postzone Effect: Incorrect ratios can lead to false negatives. Excess antibody (prozone) or excess antigen (postzone) can hinder optimal lattice formation.
Technical Errors
- Under-Centrifugation: Insufficient centrifugation may not bring cells into close enough contact for agglutination.
- Failure to Observe at Proper Phase: Missing a reaction at immediate spin or after 37°C incubation.
- Incorrect Incubation Time/Temperature: Not allowing sufficient time or maintaining the correct temperature for antibody binding.
Antibody Characteristics
- Low Titer/Weak Antibodies: Some clinically significant antibodies may be present in very low concentrations, making them difficult to detect.
- Antibodies showing Dosage: Some antibodies (e.g., Anti-Fy(a), Anti-Jk(a)) react stronger with homozygous cells than heterozygous cells.
- Antibodies to Low-Frequency Antigens: If the donor cells happen to lack the specific antigen targeted by a recipient’s antibody that wasn’t identified in the initial screen.
Solution
- Enhance Sensitivity: Consider using enhancement media (e.g., LISS, PEG) if not already part of the protocol, as these can increase the sensitivity of antibody detection, particularly in the AHG phase, particularly false positives and false negatives, as these can have critical implications for patient safety during transfusions.
- Review Protocol Adherence: Meticulously re-check every step of the protocol, especially the addition of all reagents (serum, cells, AHG) and proper washing.
- Check Reagent Integrity: Verify expiry dates and proper storage conditions of all reagents. Perform quality control checks on AHG reagent using IgG-sensitized control cells (e.g., Coombs control cells). These cells must agglutinate with AHG to confirm its activity.
- Ensure Adequate Washing: Re-emphasize thorough washing (at least 3-4 times) with proper decantation.
- Optimize Cell/Serum Ratio: Ensure correct volumes are used as per the protocol.
- Optimize Centrifugation: Verify centrifuge speed and time settings.
- Check Incubation: Confirm correct temperature (e.g., 37°C) and incubation time.
- Repeat Testing: If suspicion remains, repeat the crossmatch carefully with fresh reagents and samples if possible.
- Investigate Patient History: Review the patient’s transfusion history, prior antibody screens, and diagnoses that might explain a weak or unusual reaction.
Frequently Asked Questions (FAQs)
What is the chief purpose of performing a serologic cross-matching?
The chief purpose of performing a serologic cross-matching (blood compatibility test) is to maximize the safety of blood transfusions by ensuring blood compatibility between donor red blood cells and recipient serum/plasma. It serves two primary functions.
1. Detecting ABO incompatibility: This is the most critical aspect, as mismatched ABO blood types can lead to immediate and life-threatening hemolytic reactions. The cross-matching (blood compatibility test) directly exposes donor red blood cells to the recipient’s serum, simulating an actual transfusion and revealing any ABO incompatibility that might have been missed in preliminary tests like blood typing.
2. Identifying clinically significant antibodies: These are antibodies in the recipient’s serum that can react with antigens on donor red blood cells, potentially causing delayed hemolytic reactions. The serological cross-matching (blood compatibility test) acts as a final safeguard, detecting even weak antibodies that might go unnoticed in other tests, thereby preventing these potential complications.
Major Cross-matching
- Mandatory for nearly all transfusions.
- Detects ABO incompatibility, strong irregular antibodies, and confirms overall compatibility.
Minor Cross-matching
- Less frequent, performed based on specific scenarios and institutional policies.
- Detects potential harm from donor antibodies to recipient red blood cells.
Overall, the serologic cross-matching (blood compatibility test) plays a crucial role in transfusion medicine by
- Preventing both immediate and delayed hemolytic reactions.
- Minimizing transfusion risks by verifying blood compatibility beyond routine blood typing and antibody screening.
- Offering an additional layer of safety and confidence in transfusion decisions.
What causes positive results in the serologic cross-matching?
A positive result in a serologic cross-matching (blood compatibility test) indicates that incompatibility has been detected between the donor’s red blood cells and the recipient’s serum/plasma. This incompatibility can manifest in various ways, leading to two main types of positive results.
1. ABO incompatibility: This is the most critical and potentially life-threatening cause of a positive cross-matching in a blood compatibility test. It occurs when the recipient’s ABO blood type is incompatible with the donor’s blood type. For example, an A recipient cannot receive O-positive blood due to the presence of A antigens on recipient cells that can react with anti-A antibodies in donor plasma.
2. Antibody-antigen reactions: This happens when the recipient has antibodies in their serum/plasma that can react with specific antigens on the donor’s red blood cells. These antibodies can be naturally occurring or developed from previous transfusions or pregnancies. The reaction between antibodies and antigens causes agglutination (clumping) of red blood cells, visible in the serologic cross-matching (blood compatibility test).
Here are some specific causes of positive results due to antibody-antigen reactions:
- Strong irregular antibodies: These are antibodies directed against antigens other than the ABO blood group system. Examples include Rh antibodies, Kell antibodies, and Duffy antibodies. Even weak forms of these antibodies can cause significant reactions if not detected, hence the importance of the cross-matching (blood compatibility test).
- Weak irregular antibodies: These antibodies may not always cause visible agglutination in routine antibody screening tests. However, the AHG phase of the serological cross-matching (blood compatibility test) amplifies their reaction, allowing detection and preventing potential delayed hemolytic reactions.
- Autoantibodies: In rare cases, recipients may have antibodies against their own red blood cells. These can also react with donor red blood cells, leading to a positive cross-matching (blood compatibility test).
Why is minor crossmatch important?
The minor cross-matching plays a crucial role in transfusion safety even though it is not always mandatory as the major cross-matching.
Detecting Hidden Dangers
While the major cross-matching focuses on ABO incompatibility and strong antibodies, the minor cross-matching specifically targets potential risks from donor antibodies reacting with recipient red blood cells.
These donor antibodies might not be identified in routine antibody screening or might be weak, causing delayed hemolytic reactions hours or even days after the transfusion.
The minor cross-matching acts as an additional safety measure to catch these hidden dangers before they cause harm.
Crucial in Specific Situations
The importance of the minor cross-matching increases in specific scenarios like:
- Recipients with a history of transfusion reactions or known unexpected antibodies.
- Donors with rare blood types or known strong antibodies.
- Compatibility needs confirmation for blood components like platelets.
In these situations, the minor cross-matching provides valuable peace of mind and potentially prevents delayed reactions.
Complementary Roles
- Both major and minor crossmatches work together for optimal transfusion safety.
- The major cross-matching detects immediate dangers, while the minor cross-matching offers an extra layer of protection against delayed complications.
- Neglecting the minor cross-matching when potential risks exist can compromise overall safety, highlighting its significant role.
- Compared to the major cross-matching, the minor cross-matching is typically less complex and involves a shorter testing period.
What is the difference between type screen and crossmatch?
| Feature | Type and Screen | Crossmatch |
| Purpose | Determines the ABO blood type and identifies the presence of unexpected antibodies in the recipient’s serum. | Ensures compatibility between the recipient’s serum and donor red blood cells before transfusion. |
| Components Tested | Recipient’s blood only (red blood cells and serum/plasma). | Recipient’s serum/plasma and donor red blood cells. |
| Procedure | Involves ABO blood typing and antibody screening with a panel of red blood cells. | Typically involves two phases: Immediate Spin Phase and optional AHG Phase. |
| Time Taken | Relatively quick, usually completed within 30-60 minutes. | Takes longer than type and screen, usually 1-2 hours. |
| Outcome | Identifies the ABO blood type and presence of unexpected antibodies, but does not confirm blood compatibility with a specific donor unit. | Provides a definitive answer about blood compatibility between the recipient and a specific donor unit. |
| When Performed | Often done before a transfusion is needed, especially when time is not critical. | Performed immediately before a transfusion, or when there is a concern about compatibility. |
| Cost | Less expensive than crossmatch. | More expensive than type and screen. |
| Safety | Provides a basic level of safety by identifying major incompatibilities. | Offers the highest level of safety by ensuring compatibility with a specific donor unit. |
What is the difference between blood compatibility testing and cross-matching?
The terms “blood compatibility testing” and “cross-matching” are often used interchangeably in the context of blood transfusions, but there are slight nuances in their meaning and scope.
Blood Compatibility Testing
- Broader term: Encompasses all tests performed to ensure blood compatibility between recipient and donor before a transfusion.
- Includes
- Blood typing: Determining the ABO and Rh blood group of both recipient and donor.
- Antibody screening: Testing the recipient’s serum for unexpected antibodies to red blood cell antigens.
- Cross-matching: The specific test that directly mixes recipient serum/plasma with donor red blood cells to detect agglutination or other reactions indicating incompatibility.
- Overall aim: Identifies potential risks of incompatibility to maximize transfusion safety.
Cross-matching
- Specific test: Focuses on mixing recipient serum/plasma with donor red blood cells.
- Types:
- Major Cross-matching: Mandatory for most transfusions, detects ABO incompatibility, strong irregular antibodies, and overall compatibility.
- Minor Cross-matching: Performed in specific situations to detect potential harm from donor antibodies to recipient red blood cells.
- Outcome: Provides a definitive answer about blood compatibility between the specific recipient and donor involved in the transfusion.
Key Differences
- Scope: Blood compatibility testing is a broader umbrella term encompassing all procedures, while cross-matching refers to a specific test within that umbrella.
- Focus: Blood compatibility testing aims at identifying potential risks, while cross-matching directly verifies compatibility with a specific donor unit.
- Information: Blood compatibility testing provides general information about blood types and antibodies, while cross-matching offers a conclusive answer for the intended transfusion.
Why do we wash cells during cross-matching?
The main reason cells are washed during cross-matching, particularly in the AHG phase, is to remove plasma proteins and other potential interfering substances that might affect the accuracy of the test.
Benefits of Washing Cells
- Enhanced Sensitivity: Washing removes unbound antibodies and plasma proteins, allowing the AHG reagent (anti-human globulin) to specifically bind to any weak antibodies attached to red blood cells. This amplifies the reaction and makes it easier to detect even slight incompatibilities that might be missed without washing.
- Reduced Background Reactions: Certain plasma proteins can interact with red blood cells in ways that mimic antibody-antigen reactions, leading to false positives in the crossmatch. Washing eliminates these potential interfering substances, improving the precision of the cross-matching (blood compatibility test).
- Standardized Conditions: By removing variable plasma components, washing ensures a more standardized environment for the AHG reaction, allowing for consistent and reliable interpretation of cross-matching results across different units.
Types of Washes
- Saline washes: Remove plasma proteins, unbound antibodies, and other soluble elements.
- Albumin washes: In specific situations, may be used to retain some albumin while removing other potential interferents.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- American Association of Blood Banks (AABB). Technical Manual, 21st Edition, 2023.
- Dean L. Blood Groups and Red Cell Antigens [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2005.
- Bain BJ, Bates I, Laffan MA. Dacie and Lewis Practical Haematology: Expert Consult: Online and Print 12th Edition (Elsevier). 2016.
- International Society of Blood Transfusion. https://www.isbtweb.org/resources/resources-library.html
- Swarup, D., Dhot, P. S., Kotwal, J., & Verma, A. K. (2008). Comparative Study of Blood Cross Matching Using Conventional Tube and Gel Method. Medical journal, Armed Forces India, 64(2), 129–130. https://doi.org/10.1016/S0377-1237(08)80054-8
- Suhaib H. Kakamad, Mohieddin Barzegar, Mohammad Reza Rahmani. Blood Cross Matching Without Anti-Human Globulin (AHG) and Bovine Serum: A New Interest for an Old Idea. Barw Medical Journal. 2024 Nov. 25;3(1).