TL;DR
Transfusion-related acute lung injury or TRALI is an acute, life-threatening syndrome of non-cardiogenic pulmonary edema occurring during or shortly after blood transfusion. It must occur within 6 hours of completing the transfusion (typically within 1–2 hours).
Pathophysiology ▾: The “Two-Hit Model”
- First Hit (Patient): An underlying inflammatory state (e.g., sepsis, trauma, surgery) activates and primes the pulmonary endothelium and causes neutrophils to sequester in the lung capillaries.
- Second Hit (Transfusion): The blood product contains the “trigger.” This is usually:
- Antibodies: Donor anti- or anti-HNA antibodies binding to recipient neutrophils.
- Bioactive Lipids: Lipids and inflammatory mediators accumulated in stored blood products.
- Result: Activated neutrophils release toxic mediators, causing diffuse damage to the pulmonary capillary endothelium, leading to protein-rich fluid leak into the alveoli.
Clinical Presentation (The Core Triad) ▾:
- Respiratory Distress: Rapid onset of severe dyspnea and cough.
- Hypoxemia: Severe oxygen impairment, requiring supplemental oxygen or mechanical ventilation (PaO2/FIO2≤300 mmHg).
- Vitals: Most often associated with fever (transient) and hypotension.
Investigations and Diagnosis ▾:
- Diagnosis: Primarily a clinical diagnosis of exclusion.
- Chest X-ray (CXR): Shows bilateral, diffuse “fluffy” pulmonary infiltrates with a normal heart size and no signs of vascular congestion.
- Biochemical Rule-Out: BNP/NT−proBNP levels are typically normal or low, which is the critical lab test to exclude cardiogenic pulmonary edema (TACO).
- Immediate Action: Stop the transfusion immediately, maintain IV access, and notify the blood bank.
- Management: Entirely supportive. There is no specific drug therapy (steroids or diuretics are generally not helpful and may worsen hypotension).
- Respiratory Support: Aggressive oxygen therapy, non-invasive ventilation (NIV), or mechanical ventilation if needed. TRALI usually resolves within 48 to 96 hours.
- Most Effective Strategy: Exclusion of high-risk donors (primarily women with a history of multiple pregnancies) from donating plasma-rich components (FFP, apheresis platelets).
- Blood Component Selection: Preferential use of male donor plasma or nulliparous female donor plasma for FFP and Cryoprecipitate.
- Clinical Practice: Adhering strictly to transfusion guidelines to avoid unnecessary transfusions.
*Click ▾ for more information
Introduction
Transfusion-related acute lung injury (TRALI) is a severe, life-threatening adverse event following blood product transfusion, recognized as a leading cause of transfusion-related morbidity and mortality.
Characterized by the acute onset of noncardiogenic pulmonary edema and hypoxemia, transfusion-related acute lung injury (TRALI) poses a significant diagnostic and management challenge for clinicians. This article provides a comprehensive overview of transfusion-related acute lung injury (TRALI), from its historical context and evolving definitions to its complex pathophysiology, clinical presentation, and evidence-based management strategies.
Historical Perspective and Evolving Definitions
The initial description of transfusion-related acute lung injury (TRALI) emerged in the 1980s, when clinical reports highlighted a syndrome of acute respiratory distress following transfusion. The understanding of transfusion-related acute lung injury (TRALI) has since been refined through consensus conferences and the work of international bodies.
- 1980s: Early reports by Popovsky and Moore describe a syndrome of acute respiratory distress post-transfusion, initially linking it to leukocyte antibodies in donor plasma.
- 2004 International Consensus Conference: A landmark consensus conference established the definitive criteria for transfusion-related acute lung injury (TRALI). This definition is crucial for clinical diagnosis and epidemiological studies.
- Transfusion-related acute lung injury (TRALI): Acute onset of new lung injury within 6 hours of transfusion, with bilateral pulmonary infiltrates on chest radiography and a PaO2/FIO2 ratio ≤ 300 mm Hg, without evidence of circulatory overload or other ALI risk factors.
- Possible Transfusion-related acute lung injury (TRALI): Same criteria as transfusion-related acute lung injury (TRALI) but with one or more other risk factors for acute lung injury (e.g., sepsis, pneumonia, aspiration, severe trauma).
Epidemiology of Transfusion-Related Acute Lung Injury
The true incidence of transfusion-related acute lung injury (TRALI) is challenging to determine due to underdiagnosis and underreporting, but it is estimated to occur in approximately 1 in 5,000 to 1 in 10,000 transfusions of plasma-containing products.
Pathophysiology of TRALI: The “Two-Hit” Hypothesis
The most widely accepted theory for TRALI pathophysiology is the “two-hit model,” which explains how a patient’s pre-existing condition and a subsequent transfusion event work together to cause acute lung injury.
First Hit (Recipient’s Primed State)
The recipient’s underlying clinical condition (e.g., infection, trauma, surgery) leads to a state of systemic inflammation. The systemic inflammation leads to the activation of the pulmonary vascular endothelium. This activation causes the expression of adhesion molecules, which act like “sticky hooks” on the inner lining of the blood vessels. Neutrophils, which are white blood cells involved in the inflammatory response, begin to sequester or accumulate in the capillaries of the lungs, effectively “primed” for a second insult.
Second Hit (Transfused Blood Product)
This is the “trigger” that activates the primed neutrophils.
- Antibody-Mediated: This is the classic and most common theory. The donor’s plasma contains antibodies, typically against the recipient’s human leukocyte antigens ( Class II) or human neutrophil antigens (HNA). These antibodies bind to the antigens on the surface of the sequestered neutrophils. This binding activates the neutrophils, causing them to degranulate and release a powerful cocktail of inflammatory mediators.
- Bioactive Lipid-Mediated: This theory suggests that during the storage of blood products (especially older ones), bioactive lipids accumulate. When these products are transfused, the lipids directly activate the primed neutrophils that are stuck in the pulmonary capillaries, triggering the same inflammatory cascade as the antibody-mediated mechanism.
The Resulting Cascade
The activation of neutrophils by either antibodies or bioactive lipids causes them to release potent inflammatory mediators. These include cytokines, reactive oxygen species, and proteases. This release results in direct damage to the pulmonary capillary endothelium. The damage increases the permeability of the blood vessels, allowing protein-rich fluid to leak out of the capillaries and into the interstitial space and alveoli of the lungs. This fluid accumulation leads to non-cardiogenic pulmonary edema, which manifests clinically as acute respiratory distress.
Causes and Risk Factors of TRALI
The causes and risk factors for transfusion-related acute lung injury (TRALI) can be categorized based on the “Two-Hit Model,” separating the factors inherent to the patient (the First Hit) and the factors inherent to the transfused product (the Second Hit).
Transfusion Products (Source of the Second Hit)
The risk associated with the transfusion product primarily relates to the amount of donor plasma it contains, as this is where the causative antibodies or accumulated bioactive lipids reside.
Highest Risk Products
- Fresh Frozen Plasma (FFP): Contains the largest volume of plasma and is therefore the highest risk, particularly if sourced from high-risk donors.
- Platelets: Platelet concentrates often contain a significant amount of plasma and are thus frequently implicated.
- Whole Blood (less common today): Carries risk due to the plasma component.
- Cryoprecipitate: Also contains plasma, but the overall risk is lower due to smaller volume.
Lower Risk Products
- Packed Red Blood Cells (PRBCs): These are generally considered low risk, especially if they are heavily washed or leukoreduced, but some plasma inevitably remains.
The High-Risk Donor
The single most significant cause of antibody-mediated transfusion-related acute lung injury (TRALI) is the donor being the source of antibodies.
- Multiparous Women: Women who have been pregnant multiple times are at high risk for alloimmunization. Exposure to fetal blood during pregnancy can generate high-titer antibodies against paternal HLA or HNA antigens present on the recipient’s leukocytes.
- Prior Transfusion or Transplant History: Any donor who has previously received a blood transfusion or organ transplant may have developed HLA or HNA antibodies due to exposure to foreign antigens.
- Mitigation Strategy: Due to these risks, most blood centers now preferentially use plasma from male donors or nulliparous female donors (those who have never been pregnant) for FFP and plasma-rich components.
Recipient Risk Factors (The First Hit)
The “First Hit” refers to the underlying clinical state of the patient that predisposes them to lung injury by sequestering and activating neutrophils in the pulmonary capillaries.
- Critical Illness: Patients in intensive care units (ICU) are at much higher risk due to systemic inflammation.
- Sepsis/Infection: The most common priming condition. Widespread infection triggers systemic inflammatory mediators.
- Massive Trauma or Hemorrhage: Severe tissue damage releases damage-associated molecular patterns (DAMPs), which highly activate the immune system.
- Acute Lung Injury (ALI) or Acute Respiratory Distress Syndrome (ARDS): Pre-existing lung inflammation further primes the pulmonary endothelium.
- Recent Surgery: Major operations induce a generalized inflammatory state.
- Active Organ Failure: Conditions like liver failure or acute kidney injury.
The combination of an already inflamed patient (First Hit) receiving a blood product containing neutrophil-activating substances (Second Hit) is what ultimately leads to the catastrophic endothelial damage characteristic of transfusion-related acute lung injury (TRALI).
Clinical Presentation of Transfusion-Related Acute Lung Injury
Transfusion-related acute lung injury (TRALI) is a medical emergency characterized by the acute and rapid onset of respiratory distress shortly after a blood transfusion. The defining features include:
Timing
The onset is characteristic and crucial for diagnosis: within 6 hours of completing the transfusion. In severe cases, symptoms can appear dramatically faster, often within 1 to 2 hours of starting the transfusion.
Core Symptoms (The Triad)
- Rapid Dyspnea: Sudden and severe shortness of breath, often progressing rapidly to respiratory failure.
- Hypoxemia: Defined by a saturation of less than 90% on room air, or a ratio of arterial oxygen partial pressure to fraction of inspired oxygen (PaO2/FIO2) ratio of ≤300 mmHg (a criterion for Acute Lung Injury/ARDS).
- Fever and Hypotension: Patients frequently develop fever (usually >38°C) and transient or persistent hypotension shortly after the reaction begins. Hypertension is uncommon.
Physical Examination Findings
- Non-Cardiogenic Pulmonary Edema: This is the key physical finding. Auscultation often reveals bilateral pulmonary rales (crackles) due to fluid accumulation in the alveoli.
- Frothy Sputum: May be present, indicating severe pulmonary edema.
- Absence of Circulatory Overload: Crucially, unlike TACO, signs of left atrial hypertension or circulatory overload are absent. The neck veins are typically flat (low or normal jugular venous pressure, JVP), and there are no signs of peripheral edema or hepatomegaly. This distinction is vital for management.
Laboratory and Imaging Investigations
No single laboratory test or imaging study is pathognomonic for transfusion-related acute lung injury (TRALI). Investigations serve to confirm hypoxemia and rule out cardiogenic causes of pulmonary edema.
Laboratory Tests
- Arterial Blood Gas (ABG) Analysis: Essential for quantifying the severity of respiratory failure. It reveals severe hypoxemia (PaO2 low) and a widened alveolar-arterial (A−a) gradient. Initially, patients may show respiratory alkalosis (due to hyperventilation), which can progress to respiratory acidosis if fatigue and failure ensue.
- Biochemical Markers (Rule-Out): There are no specific diagnostic markers for transfusion-related acute lung injury (TRALI). Blood tests are used to exclude Transfusion-Associated Circulatory Overload (TACO) and other conditions.
- B-type Natriuretic Peptide (BNP) or N-terminal pro-BNP (NT−proBNP): Typically normal or low in transfusion-related acute lung injury (TRALI), as opposed to being significantly elevated in TACO or cardiogenic pulmonary edema.
- Cardiac Enzymes: Should be normal, helping to exclude acute myocardial infarction as a cause of pulmonary edema.
- General Inflammation: Non-specific markers like White Blood Cell (WBC) count and C-Reactive Protein (CRP) may be elevated but reflect the patient’s underlying “First Hit” inflammatory condition.
- Post-Event Antibody Testing: While not used for acute diagnosis, post-transfusion testing of the donor’s plasma and recipient’s blood for HLA and HNA antibodies is critical for confirmation and blood bank prevention strategies.
Imaging
- Chest X-ray (CXR): The mandatory imaging requirement for diagnosis.
- Findings: Classically shows bilateral, diffuse pulmonary infiltrates that appear rapidly. The infiltrates are described as “fluffy” or patchy and are indistinguishable from those seen in acute respiratory distress syndrome (ARDS).
- Key Differentiating Feature: Crucially, the heart size is usually normal, and there is typically no evidence of cardiomegaly or pleural effusions, which are often seen in fluid overload (TACO).
- CT Scan: Not routinely required but can confirm the presence of diffuse alveolar and interstitial edema and rule out other lung pathologies.
Diagnosis
The diagnosis of transfusion-related acute lung injury (TRALI) is clinical, based on the above symptoms and exclusion of other causes of acute respiratory failure, such as TACO or sepsis. The consensus criteria require all of the following:
- Acute onset
- Hypoxemia
- Bilateral pulmonary infiltrates on chest X-ray, and
- No evidence of circulatory overload.
Differential Diagnosis
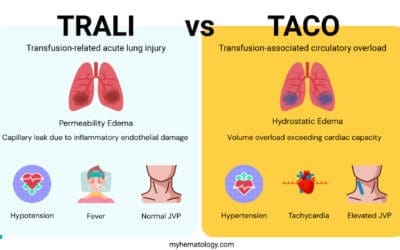
Rapid and accurate distinction of transfusion-related acute lung injury (TRALI) from other acute transfusion reactions is critical, as treatment approaches differ significantly. The most vital differential diagnosis is Transfusion-Associated Circulatory Overload (TACO).
TRALI vs. TACO (Transfusion-Associated Circulatory Overload)
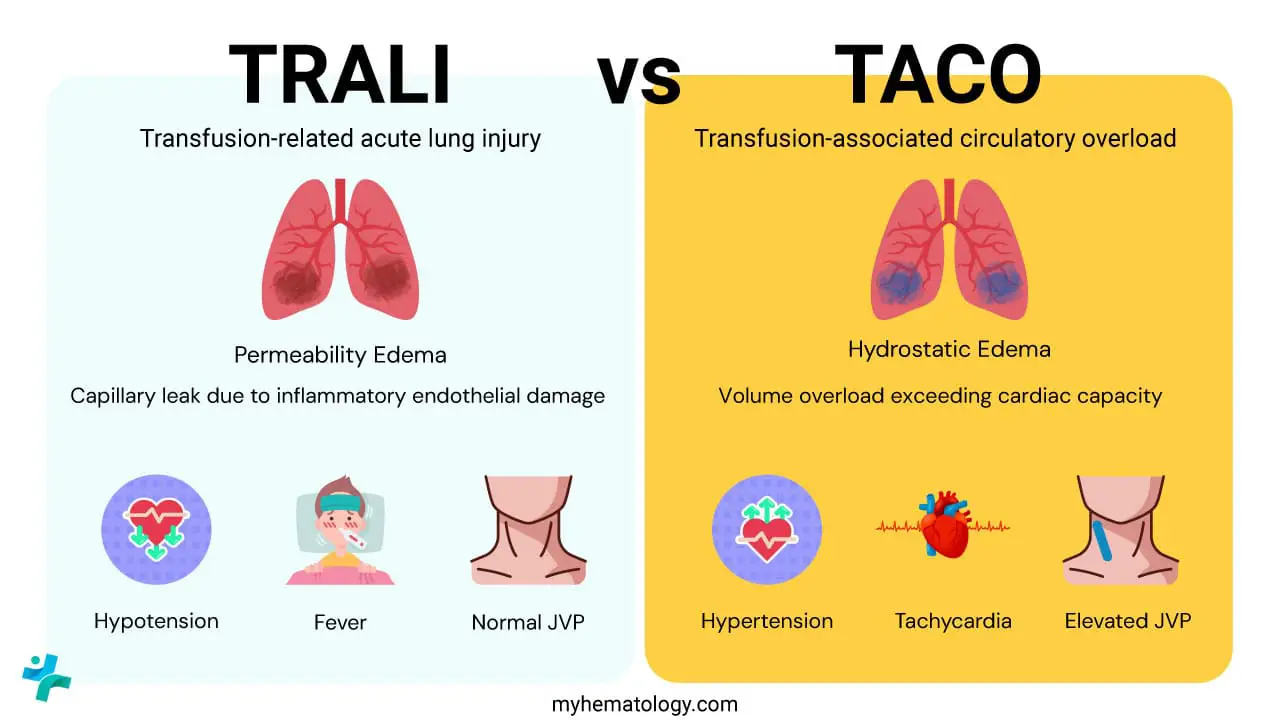
Both transfusion-related acute lung injury (TRALI) and TACO present with acute dyspnea and hypoxemia within six hours of transfusion, making clinical differentiation challenging. However, their underlying mechanisms and clinical signs are fundamentally opposite.
| Feature | TRALI (Transfusion-Related Acute Lung Injury) | TACO (Transfusion-Associated Circulatory Overload) |
| Pathophysiology | Permeability Edema (Non-Cardiogenic). Capillary leak due to inflammatory endothelial damage. | Hydrostatic Edema (Cardiogenic). Volume overload exceeding cardiac capacity. |
| Timing | Within 6 hours. | Within 6 hours (sometimes longer in less severe cases). |
| Key Vital Signs | Hypotension and Fever are common. | Hypertension and Tachycardia are common. |
| Fluid Status | Euvolemic or Hypovolemic. Low or normal. No peripheral edema. | Hypervolemic. Elevated JVP, pedal edema, bounding pulses. |
| Chest X-ray | Bilateral infiltrates. Normal heart size. No pulmonary vascular congestion. | Bilateral infiltrates. Cardiomegaly, vascular congestion, possible pleural effusions. |
| Biochemical Marker | BNP/NT−proBNP is typically Normal or Low. | BNP/NT−proBNP is typically Elevated. |
| Response to Diuretics | Poor or none, may worsen hypotension. | Excellent, leading to clinical improvement. |
| Treatment Focus | Respiratory support (Oxygen, Ventilation). No diuretics. | Fluid removal (Diuretics) and respiratory support. |
Other Important Transfusion Reactions
It is also necessary to distinguish transfusion-related acute lung injury (TRALI) from other common or severe transfusion reactions.
- Severe Allergic/Anaphylactic Reaction: Transfusion-related acute lung injury (TRALI) involves lung injury, while anaphylaxis often includes bronchospasm (wheezing), urticaria (hives), and angioedema. Anaphylaxis is a generalized IgE-mediated reaction involving mast cell degranulation, whereas transfusion-related acute lung injury (TRALI) is neutrophil-mediated lung injury. Hypotension is common in both, but respiratory findings differ.
- Acute Hemolytic Transfusion Reaction (AHTR): AHTR is caused by ABO incompatibility leading to rapid red cell destruction. It is characterized by hemoglobinuria, flank pain, and jaundice, in addition to fever and hypotension. Labs show a positive direct antiglobulin test (DAT) and signs of hemolysis (e.g., elevated lactate dehydrogenase, LDH, and indirect bilirubin).
- Febrile Non-Hemolytic Transfusion Reaction (FNHTR): FNHTR is common and mild, characterized solely by fever, chills, and mild dyspnea, usually without severe hypoxemia or radiologic evidence of pulmonary edema. Symptoms respond rapidly to antipyretics and are not life-threatening.
Management of Transfusion-Related Acute Lung Injury
Management of transfusion-related acute lung injury (TRALI) is primarily supportive, with the goal of maintaining adequate oxygenation and blood pressure while the lung injury resolves.
- Immediate Actions: Stop the transfusion immediately. Inform the blood bank and the attending physician.
- Respiratory Support: Supplemental oxygen therapy is the first step. Patients often require non-invasive or invasive mechanical ventilation to manage severe hypoxemia and respiratory distress. Lung-protective ventilation strategies are essential to minimize further lung injury.
- Hemodynamic Support: Hypotension should be managed with vasopressors. Fluid administration should be avoided unless the patient is in frank hypovolemic shock, as it can worsen pulmonary edema.
- Pharmacological Interventions: There is no definitive pharmacological treatment for transfusion-related acute lung injury (TRALI). Steroids, diuretics, and other therapies have not been shown to be consistently effective in clinical trials and are not routinely recommended.
- Prognosis: The prognosis for transfusion-related acute lung injury (TRALI) is generally good, with most patients recovering within 48 to 96 hours. However, mortality rates can be significant, ranging from 5% to 10% in severe cases.
Prevention
Prevention is the cornerstone of managing transfusion-related acute lung injury (TRALI). Prevention efforts focus primarily on reducing the transmission of antibodies and other biological response modifiers (like bioactive lipids) found in plasma-containing blood products. These strategies have significantly lowered the incidence of transfusion-related acute lung injury (TRALI) globally.
Donor Screening and Deferral (Targeting Antibodies)
This is the most effective single strategy to prevent antibody-mediated transfusion-related acute lung injury (TRALI).
Exclusion of High-Risk Donors
The primary focus is on donor populations that are likely to have developed high-titer or HNA antibodies due to exposure to foreign antigens.
- Multiparous Women: Women who have been pregnant multiple times are at high risk for alloimmunization (developing antibodies against paternal antigens present on fetal leukocytes). Most blood centers now exclude plasma-rich components (like FFP and apheresis platelets) from women who have had two or more pregnancies.
- Previous Transfusion Recipients: Donors with a history of prior blood transfusion or organ transplant may also be deferred from donating plasma-rich products.
Source Plasma Policy
Many blood services have implemented policies restricting the use of Fresh Frozen Plasma (FFP) and Cryoprecipitate to plasma sourced only from male donors or nulliparous female donors (those who have never been pregnant).
Blood Product Modification (Targeting Bioactive Lipids and Cells)
Strategies aimed at reducing the non-antibody-mediated pathway (the bioactive lipid theory).
- Leukoreduction: Filtration of blood components to reduce the number of white blood cells (leukocytes) is mandatory in many countries. While primarily intended to prevent febrile non-hemolytic transfusion reactions and CMV transmission, it is theorized to also reduce the accumulation of bioactive lipids that form during storage, as these lipids are often derived from cell breakdown.
- Limiting Storage Age: Using fresh blood products (especially platelets) may theoretically reduce the accumulation of bioactive lipids, though definitive protocols on storage age limits specifically for transfusion-related acute lung injury (TRALI) prevention are less universally mandated than donor restrictions.
Hospital and Clinical Protocols
Reducing risk at the point of care involves careful decision-making regarding transfusion necessity and speed.
- Judicious Transfusion Practice: The best prevention is avoiding unnecessary transfusions. Clinicians must strictly adhere to evidence-based guidelines for transfusion triggers.
- Prompt Reporting: Healthcare providers must be vigilant in recognizing and promptly reporting suspected transfusion-related acute lung injury (TRALI) cases to the Transfusion Service (Blood Bank). This triggers an investigation that allows the implicated blood unit and donor to be permanently deferred, preventing future reactions.
- Single Donor Products: Where possible, especially for critically ill patients, using products derived from a single donor (like apheresis platelets) minimizes exposure to different donor antibodies.
Frequently Asked Questions (FAQs)
What is the single most important diagnostic difference between TRALI and TACO?
The most critical difference lies in the underlying pathophysiology and fluid status. Transfusion-related acute lung injury (TRALI) is characterized by noncardiogenic pulmonary edema caused by inflammatory damage to the alveolar-capillary membrane, often accompanied by hypotension. TACO is characterized by cardiogenic pulmonary edema due to circulatory volume overload, typically presenting with signs of heart failure, such as elevated central venous pressure (CVP) and often hypertension. The BNP/NT-proBNP level is usually normal in transfusion-related acute lung injury (TRALI) and elevated in TACO.
Which blood products carry the highest risk of causing transfusion-related acute lung injury (TRALI)?
Any blood product containing significant amounts of plasma carries a risk, as plasma is the vehicle for the causative donor antibodies (HLA/HNA). This includes Fresh Frozen Plasma (FFP), Platelet concentrates, and sometimes Packed Red Blood Cells (PRBCs) (especially those with residual plasma). This risk has been significantly mitigated by preferential use of plasma from male or nulliparous female donors.
What immediate steps should a clinician take upon suspicion of transfusion-related acute lung injury (TRALI)?
The immediate, critical steps are:
- Stop the transfusion immediately.
- Maintain IV access.
- Perform a bedside assessment to stabilize the patient, including securing the airway and administering supplemental oxygen.
- Notify the blood bank/transfusion service immediately to initiate a workup of the reaction and quarantine the remaining blood product and donor sample.
- Provide supportive care, which often means initiating mechanical ventilation for respiratory failure and administering vasopressors for hypotension.
Is there a specific treatment or antidote for transfusion-related acute lung injury (TRALI)?
No, there is no specific pharmacological treatment or antidote for transfusion-related acute lung injury (TRALI). Management is purely supportive. This includes lung-protective ventilation (low tidal volume), aggressive oxygenation support, and managing hypotension with vasopressors. Diuretics are generally contraindicated unless there is coexisting TACO, as transfusion-related acute lung injury (TRALI) is not a volume-overload state.
How does the “two-hit” hypothesis explain non-immune transfusion-related acute lung injury (TRALI)?
In the two-hit hypothesis for non-immune transfusion-related acute lung injury (TRALI), the first hit remains the recipient’s underlying pro-inflammatory condition, which primes the neutrophils. The second hit is not an antibody, but the infusion of biologically active lipids and pro-inflammatory mediators (e.g., lysophosphatidylcholines) that accumulate in the blood product during prolonged storage. These mediators act directly on the primed neutrophils, triggering the same final pathway of endothelial damage and pulmonary edema.
Glossary of Medical Terms
Acute Lung Injury (ALI): A severe lung condition caused by inflammation, characterized by the sudden onset of poor oxygenation and bilateral pulmonary infiltrates. Transfusion-related acute lung injury (TRALI) is a specific type of ALI.
Alveolar-Capillary Barrier: The delicate structure in the lungs composed of the alveolar epithelium and capillary endothelium, which facilitates gas exchange. Damage to this barrier is the central event in transfusion-related acute lung injury (TRALI).
Human Leukocyte Antigen (HLA): Proteins found on the surface of most cells (leukocytes and nucleated cells) in the body. Antibodies against donor HLA are a common cause of immune-mediated transfusion-related acute lung injury (TRALI).
Human Neutrophil Antigen (HNA): Surface proteins specific to neutrophils. Antibodies against HNA are also a major cause of immune-mediated transfusion-related acute lung injury (TRALI).
Hypoxemia: An abnormally low concentration of oxygen in the blood, a defining feature of transfusion-related acute lung injury (TRALI).
Noncardiogenic Pulmonary Edema: Fluid accumulation in the lungs not caused by elevated hydrostatic pressure due from heart failure or volume overload (i.e., not cardiogenic). This differentiates transfusion-related acute lung injury (TRALI) from TACO.
PaO2/FiO2 Ratio: The ratio of arterial oxygen partial pressure (PaO2) to the fraction of inspired oxygen (FiO2). A ratio ≤300 mm Hg is a key diagnostic criterion for transfusion-related acute lung injury (TRALI), indicating impaired gas exchange.
Primed Neutrophils: Neutrophils that have been activated or sensitized by systemic inflammation (the “first hit”). They are ready to degranulate and cause tissue damage upon exposure to a second stimulus.
Transfusion-Associated Circulatory Overload (TACO): An adverse transfusion reaction characterized by respiratory distress due to volume overload (cardiogenic pulmonary edema), often requiring aggressive diuresis. It is the primary differential diagnosis for transfusion-related acute lung injury (TRALI).
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Cho MS, Modi P, Sharma S. Transfusion-Related Acute Lung Injury. [Updated 2023 Sep 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507846/
- Toy, P., Looney, M. R., Popovsky, M., Palfi, M., Berlin, G., Chapman, C. E., Bolton-Maggs, P., & Matthay, M. A. (2022). Transfusion-related Acute Lung Injury: 36 Years of Progress (1985-2021). Annals of the American Thoracic Society, 19(5), 705–712. https://doi.org/10.1513/AnnalsATS.202108-963CME
- Roubinian N. (2018). TACO and TRALI: biology, risk factors, and prevention strategies. Hematology. American Society of Hematology. Education Program, 2018(1), 585–594. https://doi.org/10.1182/asheducation-2018.1.585
- Fang, X., Mo, C., Zheng, L., Gao, F., Xue, F. S., & Zheng, X. (2025). Transfusion-Related Acute Lung Injury: from Mechanistic Insights to Therapeutic Strategies. Advanced science (Weinheim, Baden-Wurttemberg, Germany), 12(11), e2413364. https://doi.org/10.1002/advs.202413364
- Kuriri F. A. (2025). Transfusion-related acute lung injury (TRALI): Current understanding, challenges, and future directions. Saudi medical journal, 46(8), 865–877. https://doi.org/10.15537/smj.2025.46.8.20250233
- Peters, A. L., Van De Weerdt, E. K., Goudswaard, E. J., Binnekade, J. M., Zwaginga, J. J., Beckers, E. A. M., Zeerleder, S. S., Van Kraaij, M. G. J., Juffermans, N. P., & Vlaar, A. P. J. (2018). Reporting transfusion-related acute lung injury by clinical and preclinical disciplines. Blood transfusion = Trasfusione del sangue, 16(3), 227–234. https://doi.org/10.2450/2017.0266-16
- Kuldanek, S. A., Kelher, M., & Silliman, C. C. (2019). Risk factors, management and prevention of transfusion-related acute lung injury: a comprehensive update. Expert review of hematology, 12(9), 773–785. https://doi.org/10.1080/17474086.2019.1640599