TL;DR
Natural Killer (NK) cells are cytotoxic lymphocytes of the innate immune system, providing rapid responses against infected and cancerous cells without prior sensitization.
- Morphology ▾: They are large granular lymphocytes (LGLs) characterized by abundant cytoplasm and azurophilic granules containing perforin and granzymes.
- Development ▾: They originate from hematopoietic stem cells in the bone marrow and mature under the influence of cytokines like IL-15.
- Markers: Key surface markers include CD56 (defining NK cell subsets) and CD16 (mediating ADCC); they are typically CD3-.
- Functions ▾: They exert cytotoxicity via perforin/granzyme release and Fas/FasL interaction, mediate ADCC, produce cytokines like IFN-γ, and perform immunosurveillance.
- Lifespan & Distribution ▾: They have a relatively short lifespan in circulation and are found in blood, lymphoid organs, and peripheral tissues, recirculating via specific homing mechanisms.
- Clinical Significance: They are crucial in controlling viral infections and tumors, play complex roles in autoimmunity and transplantation, and are implicated in primary immunodeficiencies.
- Abnormalities ▾: High NK cell counts (lymphocytosis) can be reactive (due to infections, inflammation) or clonal (NK cell leukemia/lymphoma). Low NK cell counts (deficiency) increase susceptibility to infections and malignancies and can be primary (genetic) or secondary (due to immunosuppression, HIV).
- Laboratory Investigations ▾: Flow cytometry is used for identification and quantification based on surface markers, while functional assays assess cytotoxicity and cytokine production.
- Immunotherapy ▾: NK cells are targets for cancer immunotherapy, with strategies including adoptive transfer, antibody enhancement (ADCC), cytokine stimulation, and CAR NK cell development.
*Click ▾ for more information
Introduction
Natural killer (NK) cells are a type of cytotoxic lymphocyte critical to the innate immune system. They provide rapid responses to viral-infected cells and tumor formation, acting at around 3 days after infection.
Overview of the Immune System
The immune system is a complex network of cells, tissues, and organs that work together to defend the body against harmful invaders like bacteria, viruses, fungi, and parasites. It’s broadly divided into two interconnected systems: the innate and adaptive immune systems.
Innate Immunity: The First Line of Defense
This system is the body’s immediate and rapid response to pathogens. However, innate immunity doesn’t target specific pathogens. It recognizes general patterns associated with invaders (like components of bacterial cell walls) and mounts a generalized attack. It doesn’t “remember” past encounters with pathogens. Each response is essentially the same.
Key Components
- Physical Barriers: Skin, mucous membranes, stomach acid.
- Cells: Phagocytes (like macrophages and neutrophils) that engulf and destroy pathogens; natural killer (NK) cells that kill infected or cancerous cells.
- Proteins: Complement proteins that enhance the immune response; inflammatory mediators like cytokines.
Adaptive Immunity: Targeted and Specific
This system takes longer to activate (days to weeks) but provides a highly specific and targeted response. It recognizes and targets specific pathogens based on unique molecules called antigens. A key feature is its ability to “remember” past infections. After an encounter with a pathogen, the adaptive immune system generates memory cells that provide long-lasting immunity. This is the basis for vaccination.
Key Players
- B lymphocytes (B cells): Produce antibodies that bind to specific antigens, neutralizing pathogens and marking them for destruction.
- T lymphocytes (T cells):
- Cytotoxic T cells: Directly kill infected cells.
- Helper T cells: Help activate other immune cells, including B cells and macrophages.
Interaction Between Innate and Adaptive Immunity
The innate and adaptive immune systems are not independent; they work together in a coordinated manner.
- Innate immune responses, such as inflammation, help to activate the adaptive immune system.
- Cells of the innate immune system, such as dendritic cells, present antigens to T cells, initiating adaptive immune responses.
- Antibodies produced by B cells (a component of adaptive immunity) can enhance the activity of innate immune cells.
In summary, the innate immune system provides a rapid, non-specific first line of defense, while the adaptive immune system provides a slower but highly specific and long-lasting response. Both systems are essential for effective immune protection.
Key Characteristics of Natural Killer Cells
- Rapid Response: NK cells are among the first immune cells to respond to infections or tumor formation. They can recognize and eliminate infected or cancerous cells without prior sensitization, providing immediate protection.
- “Missing-Self” Recognition: NK cells possess a unique ability to recognize and kill target cells that have reduced levels of MHC class I molecules on their surface. This is known as “missing-self” recognition and is a key mechanism for identifying infected or cancerous cells, as viruses and tumors often downregulate MHC class I expression to evade T cell recognition.
- Activating and Inhibitory Receptors: NK cell activity is tightly regulated by a balance of signals from activating and inhibitory receptors on their surface. This ensures that NK cells do not attack healthy cells while effectively eliminating infected or cancerous cells.
- Cytokine Production: In addition to their cytotoxic activity, NK cells also produce cytokines such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), which play important roles in immunomodulation and the adaptive immune response. These cytokines help to activate other immune cells and enhance the overall immune response.
Role in Immunosurveillance
Natural killer (NK) cells play a critical role in immunosurveillance against tumors and viral infections.
- Anti-tumor Immunity: NK cells can recognize and kill tumor cells through various mechanisms, including “missing-self” recognition and the recognition of stress ligands on tumor cells. They can also enhance the anti-tumor activity of other immune cells, such as T cells.
- Anti-viral Immunity: NK cells can recognize and kill virus-infected cells through various mechanisms, including the recognition of viral antigens and stress ligands on infected cells. They can also produce cytokines that inhibit viral replication and enhance the anti-viral activity of other immune cells.
- Immunoregulation: NK cells can also regulate the activity of other immune cells, such as dendritic cells and T cells, helping to maintain immune homeostasis and prevent excessive inflammation.
Difference from T and B cells
| Feature | NK Cells | T Cells | B Cells |
| Type of Immunity | Innate | Adaptive | Adaptive |
| Antigen Recognition | Non-specific; recognizes targets through activating/inhibitory receptors and “missing-self” | Specific; recognizes antigens presented by APCs in MHC context | Specific; recognizes antigens through B cell receptors (BCRs) |
| Activation | Various stimuli, including stress ligands, cytokines, and antibodies (ADCC) | Requires antigen presentation by APCs and co-stimulatory signals | Binding of antigen to BCRs and signals from helper T cells |
| Effector Mechanisms | Release of cytotoxic granules (perforin, granzymes); cytokine production (IFN-γ, TNF-α) | Cytotoxic T cells: kill target cells (cytotoxic granules, apoptosis); Helper T cells: secrete cytokines | Produce antibodies; neutralize pathogens; activate complement |
| MHC Restriction | Not MHC restricted | MHC restricted | Not MHC restricted |
| Memory | Traditionally no memory, but some recent evidence suggests memory-like features | Generates memory cells | Generates memory cells |
| Key Markers | CD56+, CD3- | CD3+ | CD19+, CD20+ |
Morphology of Natural Killer Cells
Natural killer (NK) cells are classified as large granular lymphocytes (LGLs) due to their distinct morphological features.
Size and Shape
- NK cells are larger than most other lymphocytes, typically measuring 10-15 μm in diameter.
- They have a round or oval shape with a relatively abundant cytoplasm.
Nucleus
- The nucleus is typically round or kidney-shaped and located eccentrically (off-center) in the cell.
- The chromatin is dense and condensed.
Cytoplasm
- The cytoplasm is abundant and contains numerous azurophilic granules.
- These granules are lysosomes containing cytotoxic proteins such as perforin and granzymes, which are essential for NK cell-mediated killing of target cells.
Granules
- The presence of azurophilic granules is a defining characteristic of natural killer (NK) cells.
- These granules are electron-dense and vary in size and shape.
- They contain a variety of proteins involved in target cell lysis, including:
- Perforin: A pore-forming protein that creates holes in the target cell membrane.
- Granzymes: Serine proteases that enter the target cell through the pores created by perforin and induce apoptosis (programmed cell death).
Cell Surface
- Natural killer (NK) cells express a variety of surface receptors that regulate their activity, including:
- CD56: A hallmark marker for NK cells.
- CD16 (FcγRIII): A receptor for the Fc portion of IgG antibodies, involved in antibody-dependent cell-mediated cytotoxicity (ADCC).
- CD3: NK cells are typically CD3- , which helps to distinguish them from T cells.
Differences in Morphology from Other Lymphocytes
| Feature | NK Cells | T or B Cells |
| Size | Large (10-15 μm) | Small (7-10 μm) |
| Nucleus | Round or kidney-shaped, eccentric, dense chromatin | Round, central, less dense chromatin |
| Cytoplasm | Abundant, numerous azurophilic granules | Less abundant, few or no granules |
| Granules | Numerous azurophilic granules (perforin, granzymes) | Few or no granules |
| Overall Appearance | Large granular lymphocyte (LGL) | Small lymphocyte |
Development of Natural Killer Cells
Natural killer (NK) cells, like other blood cells, originate from hematopoietic stem cells (HSCs) in the bone marrow. Their development is a complex process involving multiple stages of differentiation and maturation, influenced by various cytokines and transcription factors.
Natural killer (NK) cell development begins with HSCs in the bone marrow, which are pluripotent cells capable of differentiating into all types of blood cells. HSCs give rise to common lymphoid progenitors (CLPs), which are committed to the lymphoid lineage and can differentiate into T cells, B cells, and natural killer (NK) cells. CLPs differentiate into early NK cell progenitors, which express specific markers such as CD122 (IL-2/15 receptor β chain). These progenitors undergo further differentiation and maturation under the influence of various cytokines, including IL-15, which is crucial for NK cell development and survival.
Natural killer (NK) cell development proceeds through several distinct maturation stages, characterized by the expression of specific surface markers and functional capabilities.
These stages include:
- Pre-NK cells: These cells express CD56 but lack other mature NK cell markers.
- Immature NK cells: These cells begin to express inhibitory receptors such as NKG2A and activating receptors such as NKG2C.
- Mature NK cells: These cells acquire full functional capabilities, including cytotoxicity and cytokine production. They can be further divided into two main subsets based on CD56 expression:
- CD56bright NK cells: High CD56 expression, lower cytotoxicity, mainly involved in cytokine production.
- CD56dim NK cells: Lower CD56 expression, high cytotoxicity, mainly involved in killing target cells.
Natural killer (NK) cells undergo a process called “education” or “licensing,” where they learn to distinguish between self and non-self cells. This process involves interactions between NK cell receptors and MHC class I molecules on self cells. NK cells that recognize self MHC class I molecules receive inhibitory signals, preventing them from attacking healthy self cells. NK cells that do not recognize self MHC class I molecules remain “unlicensed” and are more likely to become activated and kill target cells.
While the bone marrow is the primary site of NK cell development, recent studies suggest that NK cells can also develop and mature in secondary lymphoid organs such as the spleen and lymph nodes.
Lifespan and Distribution
Natural killer (NK) cells are relatively short-lived compared to T and B cells. Studies in mice have shown that mature NK cells have a circulating half-life of about 7-10 days. Data in humans is more limited, but studies suggest a similar lifespan, with estimates ranging from a few days to a couple of weeks. The lifespan of NK cells can be influenced by various factors, including cytokines (e.g., IL-15), infections, and age.
Natural killer (NK) cells are found in various tissues throughout the body, including:
- Peripheral blood: NK cells constitute about 5-10% of circulating lymphocytes.
- Secondary lymphoid organs: Spleen, lymph nodes, and tonsils.
- Peripheral tissues: Liver, lungs, gut, and skin.
The distribution of NK cells can vary depending on their maturation stage and functional subset. CD56bright NK cells are more commonly found in secondary lymphoid organs, while CD56dim NK cells are more abundant in peripheral blood and tissues.
Reference Range (Normal Values)
Normal ranges can vary slightly between laboratories, but generally:
- Absolute counts: 50-600 cells/µL
- Percentage of lymphocytes: 5-20%
Functions of Natural Killer Cells
Natural killer (NK) cells are crucial components of the innate immune system, playing a vital role in immunosurveillance and immune responses against various threats.
Cytotoxicity
Natural killer (NK) cells are primarily known for their ability to directly kill target cells, such as virus-infected cells, tumor cells, and stressed cells. NK cells employ several mechanisms to induce target cell death:
- Perforin/granzyme pathway: Upon activation, NK cells release cytotoxic granules containing perforin and granzymes. Perforin forms pores in the target cell membrane, allowing granzymes to enter and induce apoptosis (programmed cell death).
- Fas/FasL interaction: NK cells can express Fas ligand (FasL) on their surface, which binds to Fas (a death receptor) on target cells, triggering apoptosis.
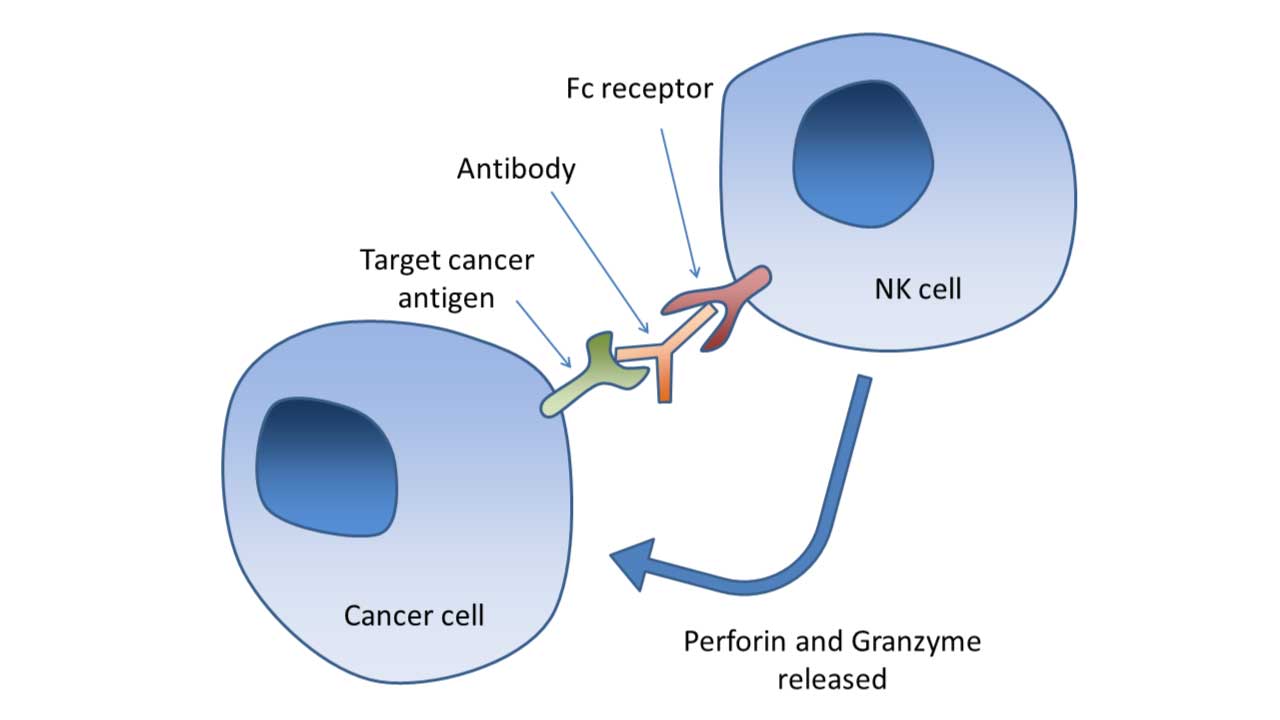
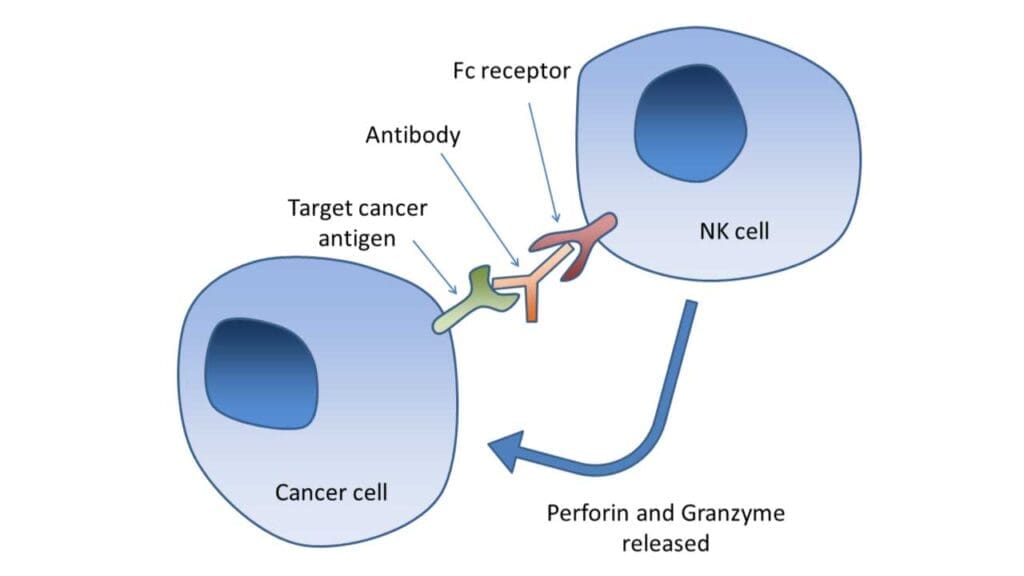
Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
Natural killer (NK) cells express CD16 (FcγRIII), a receptor for the Fc portion of IgG antibodies. This allows NK cells to recognize and kill antibody-coated target cells through ADCC. ADCC is a crucial mechanism in cancer immunotherapy, where therapeutic antibodies are used to target tumor cells and enhance NK cell-mediated killing.
Cytokine Production
Natural killer (NK) cells produce various cytokines that play important roles in regulating immune responses.
Key cytokines
- IFN-γ (interferon-gamma): A potent activator of macrophages and other immune cells, involved in antiviral and antitumor responses.
- TNF-α (tumor necrosis factor-alpha): A pro-inflammatory cytokine that can induce apoptosis in tumor cells and activate other immune cells.
- GM-CSF (granulocyte-macrophage colony-stimulating factor): Stimulates the production and differentiation of granulocytes and macrophages.
Immunosurveillance
Natural killer (NK) cells play a crucial role in immunosurveillance, constantly patrolling the body for signs of infection or malignancy. NK cells can recognize and eliminate abnormal cells, such as virus-infected cells and tumor cells, before they can cause significant damage.
Interaction with Dendritic Cells
Natural killer (NK) cells can interact with dendritic cells (DCs), which are important antigen-presenting cells that initiate adaptive immune responses. NK cells can promote the maturation and activation of DCs, enhancing their ability to present antigens to T cells and initiate adaptive immune responses.
Role in Homeostasis
Natural killer (NK) cells can help regulate immune responses and prevent excessive inflammation. Recent studies suggest that NK cells may also play a role in clearing senescent cells, which are aging cells that can contribute to tissue damage and age-related diseases.
Natural Killer Cell Abnormalities
Natural killer (NK) cell abnormalities can manifest as either increased (lymphocytosis) or decreased (deficiency) NK cell counts, each with distinct causes and clinical implications.
High Natural Killer Cell Counts (NK Cell Lymphocytosis)
Natural killer (NK) cell lymphocytosis refers to an increase in the number of NK cells in the peripheral blood. It can be broadly classified into:
- Reactive NK cell lymphocytosis: This is a secondary response to various stimuli, such as:
- Viral infections: Infections with viruses like Epstein-Barr virus (EBV), cytomegalovirus (CMV), and human immunodeficiency virus (HIV) can trigger a transient increase in NK cell numbers.
- Autoimmune diseases: Conditions like rheumatoid arthritis and systemic lupus erythematosus can be associated with elevated NK cell counts.
- Chronic inflammation: Chronic inflammatory conditions can also lead to increased NK cell numbers.
- Clonal NK cell expansions: This involves the uncontrolled proliferation of a single NK cell clone, leading to a significant increase in natural killer (NK) cell numbers. This can occur in:
- NK cell leukemia/lymphoma: These are rare types of cancer that originate from NK cells.
The clinical significance of NK cell lymphocytosis depends on the underlying cause. Reactive lymphocytosis is usually transient and resolves with the resolution of the underlying condition. Clonal expansions, on the other hand, can be indicative of malignancy and require further investigation and treatment.
Low Natural Killer Cell Counts (NK Cell Deficiency)
Natural killer (NK) cell deficiency refers to a decrease in the number or function of NK cells. It can be classified into:
- Primary NK cell deficiencies: These are rare genetic disorders that affect NK cell development or function.
- Secondary NK cell deficiencies: These are caused by external factors, such as:
- Immunosuppressive therapies: Medications used to suppress the immune system, such as those used after organ transplantation or in autoimmune diseases, can decrease NK cell numbers or function.
- HIV infection: HIV infection can lead to a decline in NK cell numbers and function.
- Other conditions: Malnutrition, certain infections, and some types of cancer can also be associated with secondary NK cell deficiencies.
Individuals with natural killer (NK) cell deficiencies are particularly susceptible to viral infections, especially herpesviruses (e.g., herpes simplex virus, varicella-zoster virus, cytomegalovirus) and human papillomavirus (HPV). Natural killer (NK) cell deficiencies can also increase the risk of developing certain types of cancer, particularly those associated with viral infections.
Laboratory Investigations
Several laboratory investigations can be used to detect and characterize natural killer (NK) cells, providing valuable information about their number, phenotype, and function. These laboratory investigations are used in various clinical settings, including:
- Diagnosis of natural killer (NK) cell disorders: To diagnose primary natural killer (NK) cell deficiencies or NK cell malignancies.
- Monitoring immune status: To assess natural killer (NK) cell numbers and function in patients with infections, autoimmune diseases, or cancer.
- Evaluating response to therapy: To monitor the effects of immunomodulatory therapies on NK cell function.
- Research studies: To investigate the role of natural killer (NK) cells in various diseases and immune responses.
Flow Cytometry
Flow cytometry is a powerful technique that uses antibodies conjugated to fluorescent dyes to identify and quantify cells expressing specific surface or intracellular markers. Flow cytometry can provide both absolute counts (cells/µL) and percentages of natural killer (NK) cells within the lymphocyte population.
In flow cytometry, natural killer (NK) cells are typically identified by their characteristic surface markers:
- CD56 (a hallmark marker for NK cells)
- CD3 (NK cells are typically CD3-)
- CD16 (FcγRIII, involved in ADCC)
Flow cytometry can also be used to identify NK cell subsets based on different levels of CD56 expression for example: CD56bright NK cells (high CD56 expression, lower cytotoxicity) or CD56dim NK cells (lower CD56 expression, high cytotoxicity).
Additional markers can be used to further characterize NK cells, such as:
- CD94, NKG2A, NKG2C (receptors involved in NK cell education and regulation)
- CD57 (marker associated with NK cell maturation and senescence)
Functional Assays
Functional assays assess the ability of NK cells to perform their effector functions, such as cytotoxicity and cytokine production.
Cytotoxicity assays: These assays measure the ability of NK cells to kill target cells. Common methods include:
- Chromium release assay: This traditional assay measures the release of radioactive chromium from lysed target cells.
- Flow cytometric cytotoxicity assays: These assays use fluorescent dyes to label target cells and measure their lysis by NK cells using flow cytometry.
Cytokine production assays: These assays measure the production of cytokines by NK cells, such as IFN-γ and TNF-α. Common methods include:
- ELISA (enzyme-linked immunosorbent assay): This method measures the concentration of cytokines in cell culture supernatants.
- Intracellular cytokine staining: This method uses flow cytometry to detect cytokines produced within NK cells.
Other Techniques
- Microscopy: Microscopic examination of stained blood smears can provide some information about NK cell morphology, but it is not a definitive method for NK cell identification or quantification.
- Molecular techniques: Molecular techniques, such as PCR (polymerase chain reaction), can be used to detect genetic abnormalities associated with NK cell disorders.
Role of NK cells in Immunotherapy
NK cells are increasingly recognized for their potential in immunotherapy, particularly in cancer treatment. Strategies to harness NK cell activity include adoptive NK cell transfer, where NK cells from a patient or healthy donor are expanded and activated ex vivo before reinfusion. Monoclonal antibodies enhance NK cell-mediated ADCC by targeting tumor-specific antigens. Cytokines like IL-2 and IL-15 stimulate NK cell proliferation and cytotoxicity. Emerging approaches involve genetically modifying NK cells with chimeric antigen receptors (CARs), similar to CAR T-cell therapy, to enhance their tumor-targeting specificity. These strategies aim to boost NK cell activity, overcome tumor evasion mechanisms, and provide effective and safe cancer treatments.
Frequently Asked Questions (FAQs)
How do NK cells recognize cancer?
NK cells employ multiple strategies to recognize cancer cells, including “missing-self” recognition, recognition of stress-induced ligands, ADCC, and the integration of activating and inhibitory signals. These mechanisms enable NK cells to effectively identify and eliminate cancer cells, highlighting their importance in cancer immunosurveillance and immunotherapy.
How do viruses avoid NK cells?
Viruses employ various strategies to evade NK cell recognition and destruction. These include:
- Modulating MHC class I: Downregulating its expression or producing MHC-like decoys to inhibit NK cell activation.
- Modulating activating ligands: Reducing their expression or producing soluble forms to block NK cell receptors.
- Interfering with NK cell signaling: Inhibiting activating pathways or producing decoy receptors for signaling molecules.
- Exploiting inhibitory signals: Expressing ligands that directly activate inhibitory receptors on NK cells.
- Establishing latency: Entering a dormant state with minimal viral antigen expression.
- Infecting immune-privileged sites: Targeting areas with suppressed immune responses.
- Examples of viral evasion strategies:
- Human cytomegalovirus (HCMV): HCMV encodes a protein called UL18 that resembles MHC class I and can bind to the inhibitory receptor ILT2 on NK cells.
- Epstein-Barr virus (EBV): EBV encodes a protein called vIL-10 that can inhibit NK cell activation and cytokine production.
- HIV: HIV can downregulate MHC class I expression and interfere with NK cell signaling.
What diseases are caused by NK cells?
While NK cells are crucial for immune defense, abnormalities in their function or number can contribute to various diseases.
1. NK Cell Deficiencies
- Primary NK cell deficiencies: These are rare genetic disorders where individuals are born with a reduced number or impaired function of NK cells. This leads to increased susceptibility to viral infections, particularly herpesviruses (like herpes simplex virus, varicella-zoster virus, cytomegalovirus) and human papillomavirus (HPV).
- Secondary NK cell deficiencies: These are more common and result from external factors that affect NK cell numbers or function. Causes include:
- Immunosuppressive therapies (e.g., after organ transplant)
- HIV infection
- Malnutrition
- Certain cancers
2. NK Cell Leukemia/Lymphoma
These are rare and aggressive cancers that originate from NK cells. They can present with various symptoms, including fever, swollen lymph nodes, and skin lesions.
3. Autoimmune Diseases
NK cells have a complex role in autoimmunity. While they can help regulate inflammation and eliminate autoreactive cells, they can also contribute to disease development in some cases.
Examples of autoimmune diseases where NK cells are implicated include:
- Type 1 diabetes
- Rheumatoid arthritis
- Systemic lupus erythematosus (SLE)
- Multiple sclerosis
4. Other Conditions
- Recurrent pregnancy loss: Abnormal NK cell activity in the uterus has been linked to recurrent pregnancy loss.
- Chronic inflammatory diseases: NK cells can contribute to chronic inflammation in conditions like inflammatory bowel disease.
Do NK cells cause inflammation?
NK cells have a complex, context-dependent role in inflammation, acting as both pro- and anti-inflammatory agents. They can promote inflammation by releasing cytokines like IFN-γ and TNF-α and through the cellular debris released during target cell lysis. Conversely, they can suppress inflammation by regulating other immune cells (like dendritic cells and macrophages) and clearing senescent cells. Their specific impact on inflammation varies depending on the disease or condition, such as autoimmunity, infection, or cancer.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. 2021 Jan 6;14(1):7. doi: 10.1186/s13045-020-01014-w. PMID: 33407739; PMCID: PMC7788999.
- Montaldo E, Del Zotto G, Della Chiesa M, Mingari MC, Moretta A, De Maria A, Moretta L. Human NK cell receptors/markers: a tool to analyze NK cell development, subsets and function. Cytometry A. 2013 Aug;83(8):702-13. doi: 10.1002/cyto.a.22302. Epub 2013 May 6. PMID: 23650273.
- Vargas-Hernández A, Forbes LR. The Impact of Immunodeficiency on NK Cell Maturation and Function. Curr Allergy Asthma Rep. 2019 Jan 19;19(1):2. doi: 10.1007/s11882-019-0836-8. Erratum in: Curr Allergy Asthma Rep. 2019 Mar 7;19(3):19. doi: 10.1007/s11882-019-0856-4. PMID: 30661124.
- Shifrin N, Raulet DH, Ardolino M. NK cell self tolerance, responsiveness and missing self recognition. Semin Immunol. 2014 Apr;26(2):138-44. doi: 10.1016/j.smim.2014.02.007. Epub 2014 Mar 12. PMID: 24629893; PMCID: PMC3984600.
- Cichocki F, Sitnicka E, Bryceson YT. NK cell development and function–plasticity and redundancy unleashed. Semin Immunol. 2014 Apr;26(2):114-26. doi: 10.1016/j.smim.2014.02.003. Epub 2014 Mar 1. PMID: 24594002.
- Domaica CI, Sierra JM, Zwirner NW, Fuertes MB. Immunomodulation of NK Cell Activity. Methods Mol Biol. 2020;2097:125-136. doi: 10.1007/978-1-0716-0203-4_9. PMID: 31776924.
- Bryceson YT, Fauriat C, Nunes JM, Wood SM, Björkström NK, Long EO, Ljunggren HG. Functional analysis of human NK cells by flow cytometry. Methods Mol Biol. 2010;612:335-52. doi: 10.1007/978-1-60761-362-6_23. PMID: 20033652; PMCID: PMC4969010.