TL;DR
Monoclonal Gammopathy of Undetermined Significance (MGUS) is an asymptomatic premalignant plasma cell or lymphoplasmacytic disorder.
- Classification ▾: Divided into Non-IgM MGUS (most common, precursor to Multiple Myeloma, MM), IgM MGUS (precursor to Waldenström Macroglobulinemia, WM), and Light Chain MGUS (LC-MGUS).
- Pathophysiology ▾: Arises from a small, stable clone of B-lymphocytes/plasma cells that have acquired primary genetic abnormalities (the “first hit”). The clone produces a structurally identical, non-functional immunoglobulin (M-protein). The overall risk of progression to symptomatic malignancy is approximately 1% per year. Progression requires the accumulation of secondary genetic events (the “second hit”).
- Signs and symptoms ▾: MGUS is asymptomatic and does not cause the myeloma-defining CRAB symptoms (HyperCalcemia, Renal insufficiency, Anemia, Bone lesions).
- The M-protein itself can cause symptoms even without malignancy. This is termed Monoclonal Gammopathy of Clinical Significance (MGCS), including conditions like peripheral neuropathy or M-protein-related nephropathy.
- Investigations and Diagnosis ▾: Diagnosis requires Serum Protein Electrophoresis (SPEP)/Immunofixation (IFE), Quantitative Immunoglobulins, and Serum Free Light Chain (FLC) assay. Bone marrow aspiration/biopsy (< 10% clonal cells) and skeletal imaging (X-ray, CT, or PET/CT) are required to confirm the absence of symptomatic disease.
- Management ▾: No active therapy. Active Surveillance (Watchful Waiting).
*Click ▾ for more information
Introduction
Monoclonal Gammopathy of Undetermined Significance (MGUS) is the most common precursor state to Multiple Myeloma (MM) and related lymphoproliferative disorders. It is defined by the presence of a circulating monoclonal protein (M-protein or paraprotein) without evidence of end-organ damage (CRAB criteria) or an underlying malignant disorder that requires treatment.
Monoclonal Gammopathy of Undetermined Significance (MGUS) is not a disease in itself, but rather a diagnostic finding. It represents a quiescent or stable proliferation of a single clone of plasma cells in the bone marrow. Plasma cells are a type of white blood cell derived from B lymphocytes, and their normal function is to produce antibodies (immunoglobulins) to fight infection.
In Monoclonal Gammopathy of Undetermined Significance (MGUS), a single clone of these plasma cells begins to multiply silently and produces a large amount of a structurally identical, non-functional antibody (the M-protein). The “Undetermined Significance” part of the name indicates that while the abnormal protein is present, the individual does not yet have any related symptoms, organ damage, or end-organ complications (such as those seen in multiple myeloma, amyloidosis, or related disorders).
Monoclonal Gammopathy of Undetermined Significance (MGUS) is a highly prevalent condition, particularly in aging populations, affecting approximately 3% of individuals over age 50 and rising to over 5% in those older than 70. Its clinical significance lies in its potential to progress to symptomatic hematologic malignancy, primarily MM, at a rate of roughly 1% per year. Given this low but persistent risk, appropriate diagnosis and long-term surveillance are paramount for clinical practice.
The Spectrum of Plasma Cell Dyscrasias
Monoclonal Gammopathy of Undetermined Significance (MGUS) exists on a recognized continuum of plasma cell disorders that eventually lead to symptomatic myeloma, highlighting the stepwise nature of oncogenesis:
MGUS → Low-Risk Progression → Smoldering Multiple Myeloma (SMM) → High-Risk Progression → Active Multiple Myeloma (MM)
MGUS can also progress to other disorders, most commonly Waldenström Macroglobulinemia (WM) (preceded by IgM-MGUS) or Light-Chain Amyloidosis (AL) (preceded by Light-Chain MGUS).
The classification is primarily based on the type of monoclonal immunoglobulin (M-protein) that is being produced by the clonal plasma cells or lymphocytes.
| Type of MGUS | M-protein/Finding | Prevalence | Primary Malignant Progression Risk |
| Non-IgM MGUS | IgG or IgA intact M-protein | ~ 85% | Multiple Myeloma (MM) or AL Amyloidosis |
| IgM MGUS | IgM intact M-protein | ~ 15% | Waldenström Macroglobulinemia (WM) or Lymphoma |
| Light Chain MGUS | Abnormal FLC ratio with increased involved free light chains; no intact M-protein | Least Common | Light Chain MM or Light Chain AL Amyloidosis |
Pathophysiology and Mechanisms of Clonal Initiation
The fundamental event in Monoclonal Gammopathy of Undetermined Significance (MGUS) is the immortalization and restricted proliferation of a single plasma cell clone in the bone marrow. This clone secretes a structurally identical immunoglobulin (the M-protein).
Monoclonal Gammopathy of Undetermined Significance (MGUS) is fundamentally a disorder of B-cell differentiation. It starts when a single B-lymphocyte, in the bone marrow, undergoes a transformation event.
During normal B-cell maturation (specifically during VDJ recombination and class-switch recombination), this single cell acquires one or more initiating genetic abnormalities. These are often the same primary lesions found in Multiple Myeloma (MM), such as:
- Hyperdiploidy: Having too many chromosomes.
- Translocations: Specific pieces of chromosomes switching places, most commonly the IgH (immunoglobulin heavy chain) locus on chromosome 14q32 with partner genes like t(11;14) or t(4;14).
These genetic changes allow the single B-cell to transform into an immortalized clone of plasma cells. However, in Monoclonal Gammopathy of Undetermined Significance (MGUS), this clone remains small (< 10% of the bone marrow cellularity) and relatively stable, often held in check by the bone marrow microenvironment.
The Role of the M-protein
The defining output of this clone is the Monoclonal protein (M-protein or paraprotein), which is an excess of a single, structurally identical immunoglobulin (like IgG, IgM, or free light chains).
- Non-Functionality: Although immunoglobulins are meant to be antibodies, the M-protein produced in MGUS is usually not functional. It is not produced in response to a specific antigen (like in a normal immune response), but rather as an unregulated overflow product of the clonal cells.
- Organ Impact (In LC-MGUS): In Light Chain MGUS (LC-MGUS), the free light chains (small portions of the antibody) can be directly toxic. Because of their size, they are filtered by the kidneys, where they can precipitate or cause damage, leading to Monoclonal Gammopathy of Clinical Significance (MGCS) related renal impairment even without full-blown myeloma.
The Bone Marrow Microenvironment
The environment within the bone marrow plays a critical role in keeping Monoclonal Gammopathy of Undetermined Significance (MGUS) in check and is essential for understanding progression.
Normal plasma cells rely on support from the bone marrow stromal cells (e.g., bone cells, fibroblasts). In Monoclonal Gammopathy of Undetermined Significance (MGUS), the clonal plasma cells interact with this environment, utilizing growth and survival factors (cytokines). Interleukin-6 (IL-6) is a historically important cytokine, but others like Vascular Endothelial Growth Factor (VEGF) are also involved in supporting the clone.
The clonal cells can subtly alter the immune landscape, often leading to suppression of the unaffected plasma cells (polyclonal immunoglobulins). This can result in a state of relative immune deficiency, which is why Monoclonal Gammopathy of Undetermined Significance (MGUS) patients are at a higher risk of infection, even though they are asymptomatic.
The Transition to Malignancy (The “Second Hit”)
Monoclonal Gammopathy of Undetermined Significance (MGUS) progresses to symptomatic diseases (like Multiple Myeloma or Waldenström Macroglobulinemia) in about 1% of patients per year. This transition requires overcoming the regulatory mechanisms, which is thought to be driven by secondary events.
- Secondary Genetic Events: Over time, the clonal cells accumulate additional genetic lesions (e.g., deletions, mutations like RAS or MYC activation). These “second hits” confer greater proliferative or survival advantage, leading to an expansion of the clone (Smoldering Multiple Myeloma, SMM) and eventually, full malignancy (MM), characterized by bone destruction, kidney failure, and other complications.
- Microenvironment Changes: The clonal cells become less dependent on the stromal cells and begin to actively manipulate the microenvironment (e.g., by activating osteoclasts to destroy bone), leading to the symptomatic organ damage that defines Multiple Myeloma (CRAB criteria).
Signs and Symptoms of Monoclonal Gammopathy of Undetermined Significance (MGUS)
By definition, Monoclonal Gammopathy of Undetermined Significance (MGUS) is asymptomatic. The diagnosis requires the absence of organ damage, specifically the classic myeloma-defining criteria, known as CRAB symptoms:
- HyperCalcemia (elevated calcium)
- Renal Insufficiency (kidney failure)
- Anemia (low red blood cell count)
- Bone Lesions (lytic lesions or holes in the bones)
If a patient with an existing Monoclonal Gammopathy of Undetermined Significance (MGUS) diagnosis develops any of these CRAB symptoms, it is highly suggestive that the condition has progressed to symptomatic Multiple Myeloma (MM) or a related malignancy.
Monoclonal Gammopathy of Clinical Significance (MGCS)
While Monoclonal Gammopathy of Undetermined Significance (MGUS) does not cause CRAB symptoms, the M-protein itself can directly interact with tissues, leading to symptoms or organ dysfunction. When this occurs, the condition is often reclassified as Monoclonal Gammopathy of Clinical Significance (MGCS), which warrants treatment directed at the underlying clonal cells, even if the clone is small.
These non-malignancy-related symptoms are caused by the pathogenicity of the M-protein, not the mass of the plasma cells.
Specific Clinical Manifestations (MGCS Symptoms)
- Peripheral Neuropathy (Monoclonal Gammopathy of Neurological Significance – MGNS): Most commonly seen with IgM MGUS, the M-protein can directly attack nerve tissues, often by binding to myelin-associated glycoprotein (MAG) on the nerve sheath. Symptoms include numbness, tingling, pain, weakness, or gait instability, typically starting in the feet and hands.
- Nephropathy (Kidney Disease): Often seen with LC-MGUS, the excess free light chains are filtered by the kidneys, where they can be deposited in or damage the renal tubules or glomeruli. Symptoms include proteinuria (excess protein in the urine), hematuria (blood in the urine), or a slow, unexplained decline in renal function (elevated serum creatinine). Examples include Light Chain Deposition Disease and C3 Glomerulopathy.
- Dermatologic and Vascular Manifestations:
- Cryoglobulinemia: The M-protein precipitates in cold temperatures, often causing painful skin rashes or vascular issues, particularly in the extremities.
- Scleromyxedema: A rare, chronic disorder causing papules and diffuse thickening of the skin, strongly associated with an IgG lambda M-protein.
- Acquired Factor X Deficiency: The M-protein interferes with a clotting factor (Factor X), leading to bleeding tendencies.
Diagnostic Criteria and Baseline Workup of MGUS
The diagnosis of MGUS is based on the consensus criteria established by the International Myeloma Working Group (IMWG), which must definitively exclude both active MM and SMM.
The IMWG Diagnostic Criteria
| Feature | Non-IgM MGUS (IgG, IgA, IgD) | IgM MGUS (Precursor to WM) | Light-Chain MGUS (LC-MGUS) |
| Serum Monoclonal Protein (M-protein) | < 30 g/L (3.0 g/dL) | < 30 g/L (3.0 g/dL) | No M-protein on SPEP and IFE |
| Bone Marrow Clonal Plasma Cells | < 10% | < 10% | < 10% |
| Serum Free Light Chain (sFLC) Ratio | N/A (Included in risk stratification) | N/A | Abnormal κ/λ ratio |
| End-Organ Damage (CRAB) | Absent | Absent | Absent |
| Related Disorders | Absent (e.g., Amyloidosis) | Absent (e.g., Lymphoma, AL Amyloidosis) | Absent |
Required Baseline Diagnostic Testing
The initial workup is critical to exclude symptomatic disease and determine the level of risk.
- Serum Studies:
- Complete Blood Count (CBC), Serum Calcium, and Creatinine (to exclude CRAB).
- Serum Protein Electrophoresis (SPEP) and Immunofixation Electrophoresis (IFE): Confirms the presence, type, and quantity of the M-protein.
- Serum Free Light Chain (sFLC) Assay and Ratio: Essential for risk stratification and detecting LC-MGUS.
- Quantitative Immunoglobulins (IgG, IgA, IgM): To assess for Immunoparesis (suppression of uninvolved Igs), an additional prognostic factor.
- Bone Marrow Evaluation: A bone marrow aspirate and biopsy is required for definitive Monoclonal Gammopathy of Undetermined Significance (MGUS) diagnosis if the M-protein is non-IgG, the M-protein concentration is ≥ 15 g/L, or the sFLC ratio is abnormal, as these factors increase the likelihood of SMM. The biopsy should include fluorescence in situ hybridization (FISH) and flow cytometry to assess clonal percentage and cytogenetics.
Risk Stratification for Progression (The Mayo Model)
The progression rate of ~ 1% per year is an average. Individualized risk assessment is vital for determining the appropriate surveillance schedule. The most widely adopted model is the Mayo Clinic MGUS Risk Stratification Model, which uses three readily available clinical parameters to predict the 20-year absolute risk of progression to symptomatic disease.
The Three Key Risk Factors
One point is assigned for the presence of each factor:
- Serum M-protein concentration ≥ 15 g/L (1.5 g/dL)
- Non-IgG M-protein isotype (i.e., IgA or IgM)
- Abnormal sFLC ratio (involved FLC to uninvolved FLC ratio outside the normal range of 0.26 to 1.65 when using the Freelite assay).
Risk Groups and Absolute Risk
| Risk Group | Number of Risk Factors | Absolute Risk of Progression at 20 Years |
| Low-Risk MGUS | 0 | 5% |
| Low-Intermediate Risk | 1 | 21% |
| High-Intermediate Risk | 2 | 37% |
| High-Risk MGUS | 3 | 58% |
Progression Risk in Subtypes
- IgG MGUS is the most common and has the lowest risk of progression to MM.
- IgA MGUS has a higher risk of progression to MM.
- IgM MGUS has a higher propensity to progress to Waldenström Macroglobulinemia (WM) or B-cell lymphoma.
- LC-MGUS is a precursor to light-chain MM or AL amyloidosis.
Differential Diagnosis of Monoclonal Gammopathy of Undetermined Significance
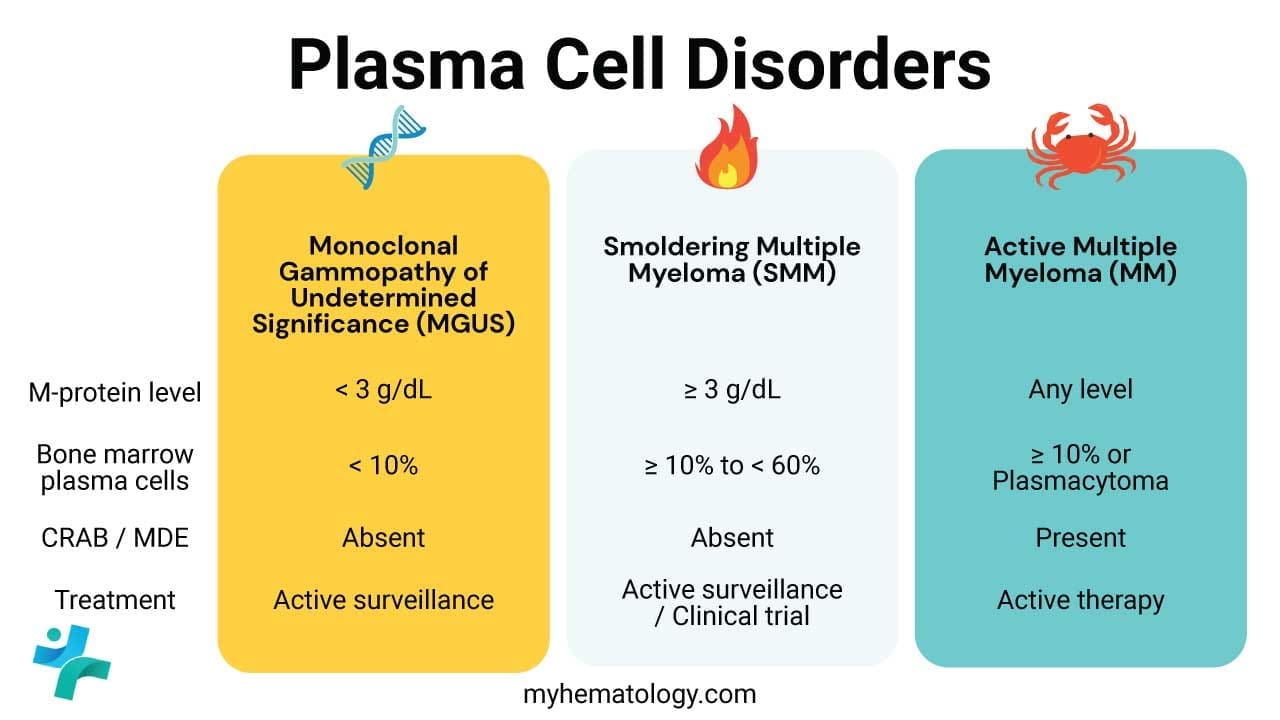
Distinguishing Monoclonal Gammopathy of Undetermined Significance (MGUS) from its related, more serious conditions is essential, as management changes from observation to active treatment. The table below outlines the key diagnostic thresholds based on the International Myeloma Working Group (IMWG) criteria.
| Condition | M-protein (M-spike) | Bone Marrow Plasma Cells (BMPC) | Symptoms / Organ Damage | Treatment |
| MGUS (Non-IgM) | < 3 g/dL | < 10% | Absent (No CRAB or MDEs) | Observation |
| Smoldering Multiple Myeloma (SMM) | ≥ 3 g/dL OR | ≥ 10% but < 60% | Absent (No CRAB or MDEs) | Observation/Clinical Trials |
| Active Multiple Myeloma (MM) | Any level | ≥ 10% (OR Plasmacytoma) | Present (≥ 1 CRAB criterion or Myeloma Defining Event (MDE)) | Active Therapy |
| Waldenström Macroglobulinemia (WM) | IgM M-protein (Any level) | Lymphoplasmacytic infiltration ≥ 10% | May be present (e.g., hyperviscosity, cytopenia, WM-related CRAB) | Active Therapy (if symptomatic) |
| AL Amyloidosis | Any M-protein (often small) | < 10% (often) | Present (Biopsy-proven amyloid deposits in organs) | Active Therapy |
Management and Surveillance Guidelines
The cornerstone of Monoclonal Gammopathy of Undetermined Significance (MGUS) management is Active Surveillance. No pharmacologic intervention is currently recommended to prevent progression. Treatment is reserved for symptomatic progression or the development of associated complications.
Monitoring Strategy by Risk Group
The frequency of follow-up is guided by the patient’s initial risk category, as per IMWG/ASH guidelines:
- Low-Risk MGUS (0 factors): Repeat SPEP and sFLC ratio at 6 months. If stable, continue monitoring with SPEP and sFLC every 2 to 3 years or only if symptoms of myeloma or related disorders arise. Bone marrow examination is typically not required at baseline unless there is an unexplained abnormality in the CBC, calcium, or creatinine.
- Low-Intermediate, High-Intermediate, and High-Risk MGUS (1–3 factors): Requires a bone marrow biopsy and skeletal imaging (CT or PET/CT is often preferred over conventional X-ray). Repeat SPEP and sFLC ratio at 6 months. If stable, continue monitoring annually for life.
Screening for Associated Complications (MGRS)
Monoclonal Gammopathy of Undetermined Significance (MGUS) is not simply a risk factor for MM; the clone itself can cause morbidity. These conditions are termed Monoclonal Gammopathy of Renal Significance (MGRS) when the monoclonal protein causes organ damage without meeting criteria for MM or SMM.
- Osteoporosis and Fracture Risk: Patients with Monoclonal Gammopathy of Undetermined Significance (MGUS) have a higher risk of vertebral and non-vertebral fractures due to alterations in the bone microenvironment, even without lytic lesions. A baseline assessment of bone mineral density (DEXA scan) should be considered, and prompt treatment with bisphosphonates or denosumab is warranted for confirmed osteoporosis.
- Increased Infection Risk: Immunoparesis is strongly associated with an increased risk of serious, recurrent bacterial infections. Clinicians must maintain a high index of suspicion and ensure appropriate vaccinations.
- Neuropathy and Autoimmune Phenomena: Monoclonal immunoglobulins can sometimes possess autoantibody activity, leading to conditions like peripheral neuropathy (especially IgM MGUS related to MAG antibodies) or cryoglobulinemia.
Frequently Asked Questions (FAQs)
Does MGUS require chemotherapy?
No. Monoclonal Gammopathy of Undetermined Significance (MGUS) is an asymptomatic, premalignant condition. Treatment with chemotherapy or other anti-myeloma agents is not indicated and would unnecessarily expose patients to toxicity. The goal is active surveillance to detect the small subset of patients who progress to symptomatic, treatable disease (MM, WM, or AL amyloidosis).
How is MGUS different from Smoldering Myeloma (SMM)?
The distinction is based on the burden of disease and the associated risk of progression. SMM is defined by a higher M-protein (≥ 30 g/L) and/or higher bone marrow plasma cells (≥ 10%, but < 60%). SMM has a significantly higher annual risk of progression to MM (~ 10% per year for the first 5 years) compared to Monoclonal Gammopathy of Undetermined Significance (MGUS) (~ 1% per year).
Should all patients with MGUS get a skeletal survey?
No. Routine full skeletal imaging (X-rays, CT, or PET) is not recommended for patients with low-risk MGUS, as the yield is low and exposure to radiation is unnecessary. Imaging (typically whole-body low-dose CT or PET/CT) is reserved for patients classified as intermediate- or high-risk, or for those presenting with unexplained bone pain to rule out myeloma-defining lytic lesions.
Is there a way to prevent MGUS from progressing?
Currently, no standard-of-care intervention has been proven to prevent the progression of MGUS to MM. However, clinical trials are actively investigating novel therapies, such as immunomodulatory agents (e.g., lenalidomide) or monoclonal antibodies, in the high-risk MGUS and SMM settings.
Glossary of Key Medical Terms
- Monoclonal Protein (M-protein): A homogeneous immunoglobulin, or fragment thereof (e.g., a light chain), produced by a single, expanded clone of plasma cells. It is the hallmark of plasma cell dyscrasias.
- CRAB Criteria: Clinical indicators of end-organ damage caused by plasma cell dyscrasia: HyperCalcemia, Renal failure, Anemia, Bone lesions. Fulfillment of one criterion signifies symptomatic Multiple Myeloma (MM).
- Immunoparesis: Suppression of the production of one or more uninvolved (polyclonal) immunoglobulins (IgG, IgA, IgM), which is a risk factor for infection and disease progression in MGUS and SMM.
- sFLC Ratio: Serum Free Light Chain Ratio (κ/λ), a critical biomarker used in diagnosis and risk stratification, reflecting the balance between clonal and polyclonal light chain production.
- Smoldering Multiple Myeloma (SMM): An intermediate, asymptomatic precursor stage characterized by a higher tumor burden (higher M-protein or bone marrow plasma cell percentage) than MGUS, carrying a significantly higher annual progression risk.
- Waldenström Macroglobulinemia (WM): An indolent lymphoplasmacytic lymphoma that is typically preceded by IgM-MGUS and is defined by the presence of a monoclonal IgM protein.
- MGRS: Monoclonal Gammopathy of Renal Significance. A term used for a plasma cell clone that is causing organ damage (most commonly the kidney) through the deposition or toxic effect of the M-protein, but does not meet criteria for MM or SMM.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Kaseb H, Annamaraju P, Babiker HM. Monoclonal Gammopathy of Undetermined Significance. [Updated 2022 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507880/
- Liu, Y., & Parks, A. L. (2025). Diagnosis and Management of Monoclonal Gammopathy of Undetermined Significance: A Review. JAMA internal medicine, 185(4), 450–456. https://doi.org/10.1001/jamainternmed.2024.8124
- Landgren O. (2021). Advances in MGUS diagnosis, risk stratification, and management: introducing myeloma-defining genomic events. Hematology. American Society of Hematology. Education Program, 2021(1), 662–672. https://doi.org/10.1182/hematology.2021000303
- Kyle, R. A., Larson, D. R., Therneau, T. M., Dispenzieri, A., Kumar, S., Cerhan, J. R., & Rajkumar, S. V. (2018). Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. The New England journal of medicine, 378(3), 241–249. https://doi.org/10.1056/NEJMoa1709974
- Hermouet, S., Bigot-Corbel, E., & Harb, J. (2023). Determination of the target of monoclonal immunoglobulins: a novel diagnostic tool for individualized MGUS therapy, and prevention and therapy of smoldering and multiple myeloma. Frontiers in immunology, 14, 1253363. https://doi.org/10.3389/fimmu.2023.1253363
- Bridoux, F., Nasr, S. H., Arnulf, B., Leung, N., Sirac, C., & Jaccard, A. (2024). Renal manifestations of MGUS. Hematology. American Society of Hematology. Education Program, 2024(1), 489–498. https://doi.org/10.1182/hematology.2024000573
- Cibeira, M. T., Rodríguez-Lobato, L. G., Alejaldre, A., & Fernández de Larrea, C. (2024). Neurological manifestations of MGUS. Hematology. American Society of Hematology. Education Program, 2024(1), 499–504. https://doi.org/10.1182/hematology.2024000665