TL;DR
What is CAR T-cell therapy? ▾
CAR T-cell treatment is a type of immunotherapy that uses a patient’s own T-cells to fight cancer. T-cells are genetically engineered to express a chimeric antigen receptor (CAR) that recognizes and targets cancer cells.
How does CAR T-cell therapy work? ▾
- T-cell Collection: T-cells are extracted from the patient’s blood.
- Genetic Engineering: T-cells are modified to express the CAR.
- Cell Expansion: Engineered T-cells are multiplied in the lab.
- Cell Infusion: Expanded T-cells are infused back into the patient.
- Cancer Cell Destruction: CAR T-cells recognize and attack cancer cells.
Advantages and Disadvantages ▾
- Advantages: High efficacy rates, durable responses, potential for long-term remission.
- Disadvantages: Severe side effects (CRS, neurotoxicity), high cost, limited availability.
Ongoing Research and Future Directions ▾
- Targeting solid tumors
- Improving T-cell persistence
- Reducing side effects
- Combination therapies
- Allogeneic CAR T-cell therapy
- Next-generation CAR T-cells
- CRISPR-Cas9 gene editing
*Click ▾ for more information
Introduction
CAR T-cells are a type of immune white blood cell that has been genetically engineered specifically to fight cancer. They are created by taking T-cells from a patient’s blood, modifying them in a laboratory to express a chimeric antigen receptor (CAR), and then infusing the modified cells back into the patient. The CAR allows the T-cells to recognize and attack cancer cells more effectively.
Cancer and Traditional Treatments: A Brief Overview
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. Traditional treatments for cancer include:
- Chemotherapy: Uses drugs to kill cancer cells. However, it often targets rapidly dividing cells, both cancerous and healthy, leading to side effects like hair loss, nausea, and weakened immune system.
- Radiation Therapy: Uses high-energy rays to kill cancer cells. While effective, it can damage healthy tissues and organs, leading to side effects like fatigue, skin irritation, and difficulty swallowing.
- Surgery: Involves the physical removal of cancerous tissue. While effective for localized cancers, it may not be suitable for advanced or metastatic cancers.
These traditional treatments have significantly improved cancer survival rates, however, they often have limitations, especially when dealing with advanced or recurrent cancers. Some cancers, such as certain types of leukemia and lymphoma, may be resistant to these treatments.
CAR T-cell treatment (therapy) emerges as a promising new approach, offering hope for patients with these challenging cancers.
Concept of CAR T-cell Treatment
Immunotherapy: Harnessing the Body’s Defense
Immunotherapy is a type of cancer treatment that uses the body’s own immune system to fight cancer. The immune system is a complex network of cells and organs that work together to protect the body from infection and disease. T-cells are a type of white blood cell that plays a key role in the immune response.
How CAR T-cell treatment differs from other forms of immunotherapy
While CAR T-cell treatment (therapy) is a type of immunotherapy, it differs from other forms in a significant way. Traditional immunotherapies, like checkpoint inhibitors, work by releasing the brakes on the immune system, allowing it to recognize and attack cancer cells more effectively.
In contrast, CAR T-cell treatment (therapy) involves a more direct approach. T-cells are extracted from the patient’s blood, genetically engineered to express a chimeric antigen receptor (CAR), and then infused back into the patient. This CAR allows the T-cells to specifically recognize and target cancer cells, leading to their destruction.
Here’s a table summarizing the key differences:
| Feature | Traditional Immunotherapy (e.g., Checkpoint Inhibitors) | CAR T-cell Therapy |
| Mechanism of Action | Releases the brakes on the immune system, allowing it to attack cancer cells | Engages the immune system by directly targeting cancer cells with engineered T-cells |
| Cell Engineering | No cell engineering required | T-cells are genetically modified to express a CAR |
| Target | Cancer cells and their surrounding microenvironment | Specific antigens on the surface of cancer cells |
By understanding these distinctions, we can appreciate the unique potential of CAR T-cell therapy in revolutionizing cancer treatment.
How CAR T-Cell Treatment (Therapy) Works
Role of T-cells in the Immune System
T-cells play a crucial role in the immune system, serving as the body’s defense against infection and disease. They are a type of white blood cell that originates in the bone marrow and matures in the thymus gland.
The primary functions of T-cells include
- Direct Attack: Some T-cells, known as cytotoxic T-cells, directly attack and destroy infected cells or cancer cells. They recognize foreign antigens on the surface of these cells and eliminate them.
- Immune Regulation: Helper T-cells coordinate the immune response by releasing signaling molecules called cytokines. These cytokines activate other immune cells, such as B cells, macrophages, and other T-cells, to fight infection.
- Immune Memory: Memory T-cells retain information about past infections, enabling the body to mount a faster and more robust immune response upon re-exposure to the same pathogen. This is the basis for long-lasting immunity and the effectiveness of vaccines.
Overall, T-cells are essential for maintaining a healthy immune system and protecting the body from various threats. Their ability to recognize and respond to specific antigens makes them a powerful tool in the fight against infection and disease.
How does it work?
CAR T-cells recognize and bind to cancer cells through a specialized receptor called a chimeric antigen receptor (CAR). This CAR is engineered into the T-cell’s surface, allowing it to identify specific proteins, or antigens, on the surface of cancer cells.
- CAR Structure: The CAR has two main parts:
- Antigen-binding domain: This part is like a lock that recognizes a specific key, in this case, the antigen on the cancer cell. It’s derived from a monoclonal antibody that binds to the target antigen.
- T-cell signaling domain: This part triggers the T-cell’s activation and killing mechanisms once the CAR binds to the antigen.
- Recognition and Binding: When the CAR T-cell encounters a cancer cell displaying the target antigen, the antigen-binding domain binds to the antigen. This binding event initiates a signaling cascade within the T-cell.
- T-cell Activation: The signaling domain of the CAR transmits signals to the T-cell’s interior, activating it. This activation leads to:
- Proliferation: The T-cell starts to multiply, generating a large army of CAR T-cells to fight the cancer.
- Cytotoxicity: The T-cell releases cytotoxic molecules, such as perforin and granzyme, that can directly kill the cancer cell.
- Cytokine Release: The T-cell secretes cytokines, signaling molecules that recruit other immune cells to the tumor site and enhance the overall immune response.
By targeting specific antigens on cancer cells, CAR T-cells can effectively identify and eliminate these cells, leading to significant improvements in cancer treatment.
The CAR T-Cell Therapy Process
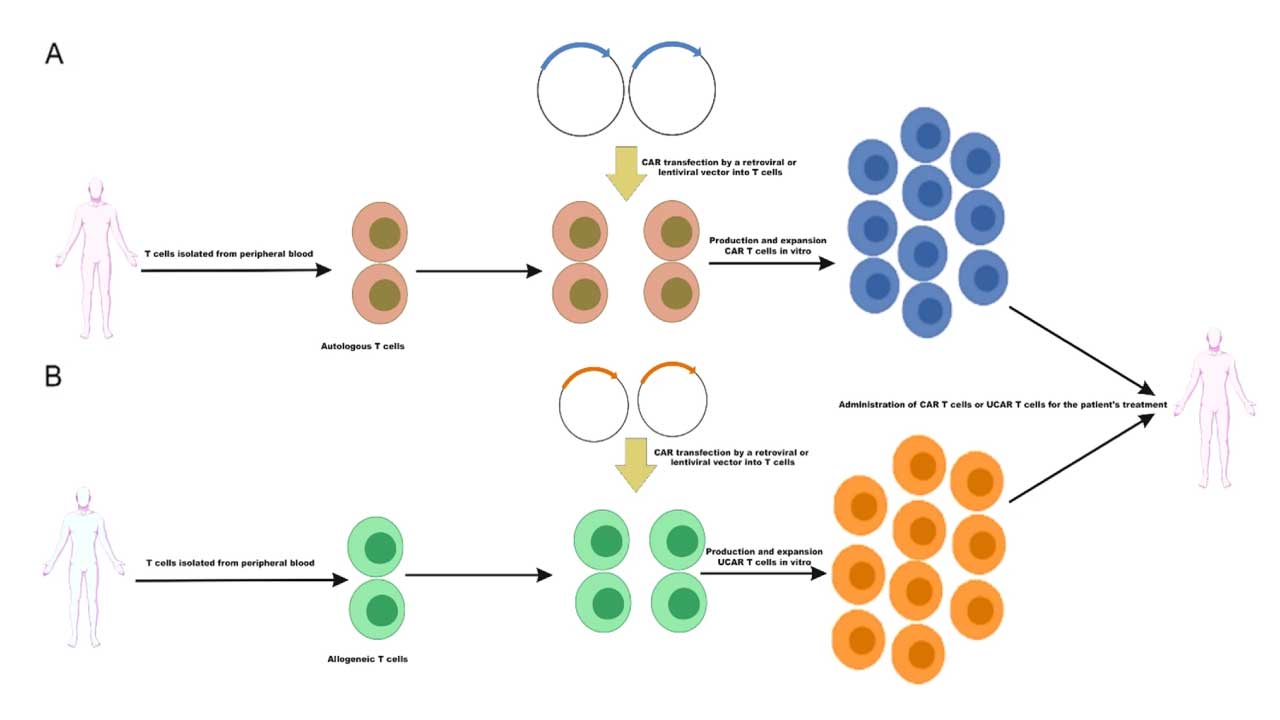
1. Patient Selection
- Disease Type and Stage: CAR T-cell treatment (therapy) is currently approved for specific types of blood cancers, such as certain types of leukemia and lymphoma. Patients with advanced or relapsed disease who have not responded to other treatments are often considered.
2. T-Cell Collection
- Apheresis: This is a procedure similar to blood donation where the patient’s blood is drawn, passed through a machine that separates the T-cells, and then the remaining blood is returned to the patient.
3. T-Cell Engineering
- Genetic Modification: The collected T-cells are genetically engineered in a laboratory to express a chimeric antigen receptor (CAR) on their surface. This CAR allows the T-cells to recognize and bind to specific antigens on cancer cells.
- Cell Expansion: The engineered T-cells are then cultured and expanded in the laboratory to increase their numbers.
4. T-Cell Infusion
- Infusion: Once a sufficient number of CAR T-cells have been generated, they are infused back into the patient’s bloodstream through a vein.
5. T-Cell Activation and Cancer Cell Destruction
- Recognition: The infused CAR T-cells circulate in the bloodstream and recognize cancer cells displaying the specific antigen.
- Binding: The CAR on the T-cell binds to the antigen on the cancer cell.
- Activation: This binding triggers the T-cell to activate and release cytotoxic molecules that kill the cancer cell.
- Proliferation / Expansion: The activated T-cells also multiply, creating a larger army of CAR T-cells to continue fighting the cancer.
6. Monitoring and Follow-up
- Side Effects: Patients are closely monitored for potential side effects, such as cytokine release syndrome (CRS) and neurological toxicities.
- Response Assessment: Regular follow-up appointments and scans are used to assess the effectiveness of the therapy and monitor for any disease progression.
It’s important to note that CAR T-cell therapy is a complex and individualized treatment. The specific process and timing may vary depending on the type of cancer and the patient’s overall health
FDA-Approved CAR T-cell Treatment
| Generic Name | Brand Name | Target Antigen | Targeted Disease | Patient Population |
| Tisagenlecleucel | Kymriah | CD19 | B-cell acute lymphoblastic leukemia (ALL) | Children and young adults with relapsed or refractory ALL |
| Axicabtagene ciloleucel | Yescarta | CD19 | Diffuse large B-cell lymphoma (DLBCL) | Adults with relapsed or refractory DLBCL |
| Brexucabtagene autoleucel | Tecartus | CD19 | Mantle cell lymphoma (MCL) | Adults with relapsed or refractory MCL |
| Lisocabtagene maraleucel | Breyanzi | CD19 | Large B-cell lymphoma | Adults with relapsed or refractory large B-cell lymphoma |
| Idecabtagene vicleucel | Abecma | BCMA | Multiple myeloma | Adults with relapsed or refractory multiple myeloma |
| Ciltacabtagene autoleucel | Carvykti | BCMA | Multiple myeloma | Adults with relapsed or refractory multiple myeloma |
Taken from https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
Advantages and Disadvantages of CAR T-Cell Treatment
| Advantages | Disadvantages |
| High response rates in certain types of blood cancers | Limited efficacy against solid tumors with risk of relapse |
| Potential for long-lasting remissions | Can lead to serious side effects like cytokine release syndrome (CRS) and neurotoxicity thus requires careful monitoring and management of side effects |
| Potential for broader applications in various cancers | High cost of treatment causes it to be not accessible to all patients |
| Ongoing research to improve efficacy and reduce side effects | Complex manufacturing process which requires specialized expertise and facilities |
| Limited to specific patient populations as it is not suitable for all cancer types |
Ongoing Research and Clinical Trials
The field of CAR T-cell therapy is rapidly evolving, with ongoing research and clinical trials exploring new strategies to improve its efficacy and safety. Some key areas of focus include:
- Targeting Solid Tumors: While CAR T-cell therapy has shown promise in treating blood cancers, extending its application to solid tumors remains a major challenge. Researchers are exploring ways to engineer CAR T-cells to target specific antigens on solid tumor cells and overcome the challenges associated with the tumor microenvironment.
- Improving T-cell Persistence: Strategies are being investigated to enhance the persistence of CAR T-cells in the body, leading to more durable responses. This includes genetic modifications to improve T-cell survival and function.
- Reducing Side Effects: Efforts are underway to develop methods to mitigate the severe side effects of CAR T-cell therapy, such as cytokine release syndrome (CRS) and neurotoxicity. This involves exploring novel CAR designs, combination therapies, and targeted interventions.
- Combination Therapies: Combining CAR T-cell therapy with other treatments, such as chemotherapy, radiation therapy, or other immunotherapies, may enhance its efficacy and overcome resistance mechanisms.
- Allogeneic CAR T-cell Therapy: Developing “off-the-shelf” allogeneic CAR T-cells derived from healthy donors can simplify the manufacturing process and potentially reduce treatment costs.
Potential Advancements in CAR T-cell Technology
- Next-Generation CAR T-cells: Researchers are exploring innovative CAR designs, such as armored CAR T-cells and universal CAR T-cells, to enhance their potency and specificity.
- Synthetic Biology: Advances in synthetic biology can be used to engineer T-cells with enhanced functions, such as the ability to secrete additional therapeutic molecules.
- CRISPR-Cas9 Gene Editing: This powerful gene-editing tool can be used to precisely modify T-cells, enabling the creation of more effective and safer CAR T-cell therapies.
By addressing these challenges and leveraging ongoing research, CAR T-cell therapy has the potential to revolutionize the treatment of cancer and provide hope for patients with previously limited treatment options.
Frequently Asked Questions (FAQs)
Is CAR T-cell therapy a gene therapy?
Yes, CAR T-cell therapy is a type of gene therapy. It involves genetically modifying a patient’s T-cells to express a chimeric antigen receptor (CAR), which allows them to recognize and attack cancer cells. This genetic modification is a key aspect of the therapy, making it a form of gene therapy.
What is the success rate of CAR T-cell treatment?
The success rate of CAR-T cell therapy can vary depending on the specific type of cancer and the individual patient. However, it has shown promising results, especially for certain blood cancers like leukemia and lymphoma.
In some cases, CAR-T cell therapy has led to significant improvements in patient outcomes, including complete remission. However, it’s important to note that not all patients respond to the treatment, and some may experience side effects.
Is CAR T-cell therapy better than chemotherapy?
CAR T-cell therapy and chemotherapy are two different approaches to cancer treatment.
While chemotherapy targets rapidly dividing cells, both healthy and cancerous, CAR T-cell therapy is more targeted, using engineered T-cells to specifically attack cancer cells.In certain cases, especially for blood cancers, CAR T-cell therapy has shown higher efficacy and longer-lasting remission compared to traditional chemotherapy. However, both treatments have their own set of side effects and are not suitable for all types of cancer or patients. The best approach depends on the individual patient’s circumstances and should be determined by a healthcare professional. Sources and related content
Is CAR T-cell therapy permanent?
No, CAR T-cell therapy is not a permanent cure for cancer. While it can lead to long-lasting remissions, it’s not guaranteed to eradicate all cancer cells. Some patients may experience a relapse after initial treatment. However, ongoing research is focused on improving the persistence of CAR T-cells in the body and developing strategies to prevent relapse.
What happens if CAR T-cell therapy fails?
If CAR T-cell therapy fails to achieve the desired outcome, patients may have several options depending on the specific circumstances and the type of cancer. These options could include:
- Additional Cycles of CAR T-cell Therapy: In some cases, additional cycles of CAR T-cell therapy may be considered, especially if there are signs of residual disease.
- Other Immunotherapy Treatments: Other immunotherapy treatments, such as checkpoint inhibitors, may be explored as alternative options.
- Chemotherapy or Radiation Therapy: Traditional treatments like chemotherapy or radiation therapy may be used in combination with or after CAR T-cell therapy.
- Clinical Trials: Participating in clinical trials can provide access to experimental therapies or new treatment combinations.
It’s important to consult with a healthcare provider to discuss the best course of action after CAR T-cell therapy, as the optimal approach will vary depending on the individual patient’s situation.
How long do CAR T-cells stay in the body?
Typically, infused CAR T-cells can persist in the body for months to years after treatment. However, their persistence may decline over time. Some CAR T-cells may remain in the body as memory T-cells, providing long-term surveillance against cancer recurrence.
How many times do you get CAR T-cell therapy?
Typically, CAR T-cell therapy is administered once. However, in some cases, additional cycles of treatment may be considered if there is evidence of persistent disease or relapse. The decision to administer multiple cycles is made on a case-by-case basis and is often based on careful evaluation by the treating physician.
Can cell surface markers on immune cells be manipulated to enhance their anti-tumor activity in CAR T-cell therapy for leukemias?
Absolutely! Manipulating surface markers on immune cells, particularly in the context of CAR T-cell therapy, holds immense potential for enhancing their anti-tumor activity in leukemias.
Current Challenges
- Limited tumor recognition: CAR T-cells are often designed to target a single marker, which can lead to tumor escape due to antigen loss or heterogeneity.
- T cell exhaustion: The harsh tumor microenvironment can exhaust CAR T-cells, reducing their efficacy and persistence.
- On-target, off-tumor toxicity: Targeting certain markers might also affect healthy tissues expressing the same marker, leading to side effects.
Strategies for Manipulation
- Multi-targeted CAR T-cells: Engineering CAR T-cells to recognize multiple tumor-specific markers simultaneously can address heterogeneity and prevent antigen escape.
- Co-stimulatory molecules: Introducing additional signaling molecules alongside the CAR can enhance T-cell activation and persistence, overcoming exhaustion.
- Suicide genes: Incorporating genes that can selectively eliminate CAR T-cells if needed minimizes risks associated with on-target, off-tumor toxicity.
- Checkpoint inhibitors: Combining CAR T-cell therapy with drugs that block inhibitory signals in the tumor microenvironment can reignite T-cell activity and improve efficacy.
- Arming CAR T-cells: Modifying CAR T-cells to secrete cytokines or cytotoxic molecules can directly increase their tumor-killing potential.
Emerging Technologies
- CRISPR-Cas9: Precisely editing genes within T-cells allows for targeted manipulation of surface markers or signaling pathways to enhance their anti-tumor properties.
- Nanoparticle-based delivery: Delivering therapeutic molecules or genetic instructions specifically to CAR T-cells can further improve their functionality and efficacy.
Considerations and Future Directions
Ethical considerations: Careful discussion and ethical frameworks are necessary as personalized medicine based on surface marker manipulation raises complex questions.
Safety and efficacy: Balancing optimal tumor targeting with minimizing potential side effects remains a critical consideration.
Clinical translation: Translating pre-clinical success into effective and personalized therapies for patients requires meticulous clinical trials and evaluation.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021 Apr 6;11(4):69. doi: 10.1038/s41408-021-00459-7. PMID: 33824268; PMCID: PMC8024391.
- Lin H, Cheng J, Mu W, Zhou J, Zhu L. Advances in Universal CAR-T Cell Therapy. Front Immunol. 2021 Oct 6;12:744823. doi: 10.3389/fimmu.2021.744823. PMID: 34691052; PMCID: PMC8526896.
- Dabas P, Danda A. Revolutionizing cancer treatment: a comprehensive review of CAR-T cell therapy. Med Oncol. 2023 Aug 22;40(9):275. doi: 10.1007/s12032-023-02146-y. PMID: 37608202.
- Zhang X, Zhang H, Lan H, Wu J, Xiao Y. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front Immunol. 2023 Feb 20;14:1101495. doi: 10.3389/fimmu.2023.1101495. PMID: 36891310; PMCID: PMC9986336.
- Hong M, Clubb JD, Chen YY. Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell. 2020 Oct 12;38(4):473-488. doi: 10.1016/j.ccell.2020.07.005. Epub 2020 Jul 30. PMID: 32735779.
- Flugel CL, Majzner RG, Krenciute G, Dotti G, Riddell SR, Wagner DL, Abou-El-Enein M. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat Rev Clin Oncol. 2023 Jan;20(1):49-62. doi: 10.1038/s41571-022-00704-3. Epub 2022 Nov 23. PMID: 36418477; PMCID: PMC10278599.
- Lanitis E, Coukos G, Irving M. All systems go: converging synthetic biology and combinatorial treatment for CAR-T cell therapy. Curr Opin Biotechnol. 2020 Oct;65:75-87. doi: 10.1016/j.copbio.2020.01.009. Epub 2020 Feb 25. PMID: 32109718.
- Tao R, Han X, Bai X, Yu J, Ma Y, Chen W, Zhang D, Li Z. Revolutionizing cancer treatment: enhancing CAR-T cell therapy with CRISPR/Cas9 gene editing technology. Front Immunol. 2024 Feb 21;15:1354825. doi: 10.3389/fimmu.2024.1354825. PMID: 38449862; PMCID: PMC10914996.
- Hu Y, Zhou Y, Zhang M, Ge W, Li Y, Yang L, Wei G, Han L, Wang H, Yu S, Chen Y, Wang Y, He X, Zhang X, Gao M, Yang J, Li X, Ren J, Huang H. CRISPR/Cas9-Engineered Universal CD19/CD22 Dual-Targeted CAR-T Cell Therapy for Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia. Clin Cancer Res. 2021 May 15;27(10):2764-2772. doi: 10.1158/1078-0432.CCR-20-3863. Epub 2021 Feb 24. PMID: 33627493.