TL;DR
Fanconi Anemia (FA) is a rare, inherited genetic disorder that primarily affects the bone marrow and increases the risk of cancer.
Key Symptoms ▾
- Bone Marrow Failure: Leading to anemia, thrombocytopenia, and neutropenia.
- Congenital Abnormalities: Skeletal, renal, skin, and other organ system abnormalities.
- Increased Cancer Risk: Particularly acute myeloid leukemia and head and neck cancers.
Causes ▾
- Genetic Mutations: Inherited mutations in genes involved in DNA repair.
- Autosomal Recessive Inheritance: Both parents must carry a mutated gene for a child to inherit the disorder.
Diagnosis ▾
- Chromosome Breakage Test: Measures increased chromosomal breaks in response to DNA damage.
- Complete Blood Count (CBC): Identifies decreased blood cell counts.
- Bone Marrow Examination: Assesses bone marrow cellularity and cell morphology.
- Genetic Testing: Confirms specific gene mutations associated with FA.
Treatment and Management ▾
- Hematopoietic Stem Cell Transplantation (HSCT): To treat bone marrow failure.
- Supportive Care: Blood transfusions, growth factors, and infection prevention.
- Cancer Prevention and Treatment: Regular screening and appropriate cancer therapies.
- Congenital Abnormality Management: Surgical correction and specialized therapies.
- Genetic Counseling and Psychosocial Support: To address family planning and emotional needs.
Prognosis ▾
- Improved Survival Rates: With advancements in medical care, especially HSCT.
- Increased Cancer Risk: Lifelong risk of cancer development.
- Quality of Life: Can be significantly impacted by the disease and its complications.
*Click ▾ for more information
Introduction
Fanconi anemia (FA) is a rare, inherited genetic disorder characterized by bone marrow failure and an increased risk of developing certain types of cancer.
Historical Overview: Fanconi anemia was first described in 1927 by Guido Fanconi. It was initially recognized as a syndrome associated with anemia, thrombocytopenia, and congenital abnormalities. Over the years, research has advanced our understanding of the underlying genetic defects and the diverse clinical manifestations of Fanconi anemia.
Significance as a Rare Genetic Disorder: Fanconi anemia is a complex genetic disorder with significant implications for affected individuals and their families. Its rarity makes it a challenging condition to diagnose and manage. However, understanding the genetic basis of Fanconi anemia has opened up new avenues for research and potential therapeutic interventions.
Impact on Multiple Organ Systems: Fanconi anemia affects multiple organ systems, leading to a wide range of clinical manifestations. Some of the most common affected systems include:
- Hematopoietic System: Progressive bone marrow failure, leading to anemia, thrombocytopenia, and neutropenia.
- Skeletal System: Skeletal abnormalities, such as short stature, radial ray defects, and hypoplastic thumbs.
- Renal System: Renal abnormalities, including horseshoe kidney and malformations of the urinary tract.
- Skin: Pigmentation abnormalities, such as café-au-lait spots and hypopigmentation.
- Gastrointestinal System: Gastrointestinal malformations, such as esophageal atresia and anal atresia.
- Cardiovascular System: Cardiac defects, such as heart defects.
- Genitourinary System: Genitourinary malformations, such as hypospadias and cryptorchidism.
- Neurological System: Neurological abnormalities, such as developmental delays and intellectual disability.
Symptoms and Causes of Fanconi Anemia
Symptoms
Fanconi anemia (FA) is a complex genetic disorder that can manifest in a variety of ways. The symptoms can range from mild to severe and can affect multiple organ systems.
Hematological Symptoms
- Bone marrow failure: This is the most common and serious complication of Fanconi anemia. It leads to a decrease in the production of red blood cells, white blood cells, and platelets.
- Anemia: This results in fatigue, weakness, and pale skin.
- Thrombocytopenia: This can cause easy bruising and bleeding.
- Neutropenia: This increases the risk of infections.
Physical Abnormalities
- Skeletal abnormalities: These can include missing or malformed thumbs, radius bones, or other bones in the arms and legs.
- Short stature: Individuals with Fanconi anemia often have shorter stature than their peers.
- Skin abnormalities: These can include areas of increased or decreased pigmentation, such as café-au-lait spots or hypopigmentation.
- Kidney abnormalities: These can include malformations of the kidneys and urinary tract.
- Heart defects: These can include various heart abnormalities.
- Gastrointestinal abnormalities: These can include malformations of the esophagus, stomach, or intestines.
Increased Cancer Risk
Individuals with Fanconi anemia have a significantly increased risk of developing certain types of cancer, including:
- Acute myeloid leukemia (AML)
- Myelodysplastic syndrome (MDS)
- Head and neck cancer
- Skin cancer
Other Symptoms
- Developmental delays
- Intellectual disability
- Microcephaly (small head size)
- Hearing loss
- Eye abnormalities
It’s important to note that the severity of symptoms can vary widely among individuals with Fanconi anemia. Some people may experience only mild symptoms, while others may have severe and life-threatening complications. Early diagnosis and appropriate management can help improve the quality of life for individuals with Fanconi anemia.
Causes
Fanconi anemia (FA) is a rare inherited genetic disorder caused by mutations in specific genes. These genes are responsible for producing proteins involved in DNA repair, particularly interstrand crosslink (ICL) repair. ICLs are DNA lesions that occur when two strands of DNA are covalently linked together, preventing DNA replication and transcription.
When these genes are mutated, the DNA repair process becomes defective, leading to the accumulation of DNA damage. This DNA damage can cause various problems seen in individuals with Fanconi anemia. Mutations in at least 23 different genes have been identified as causing Fanconi anemia. These genes are collectively known as FA genes.
The inheritance pattern of FA is typically autosomal recessive, meaning that both parents must carry a mutated gene in order for their child to inherit the disorder. However, in some cases, FA can be inherited in an X-linked recessive pattern.
Laboratory Investigations
Several laboratory tests are used to diagnose Fanconi anemia (FA). These tests help identify the characteristic features of the disease, including chromosomal instability and bone marrow failure.
Chromosome Breakage Test
This test exposes cells to a DNA-damaging agent, such as diepoxybutane (DEB). In individuals with Fanconi anemia, the cells are unable to repair the DNA damage efficiently, leading to increased chromosomal breaks.
Expected Results: Increased chromosomal breaks compared to normal individuals.
Complete Blood Count (CBC) and Peripheral Blood Smear
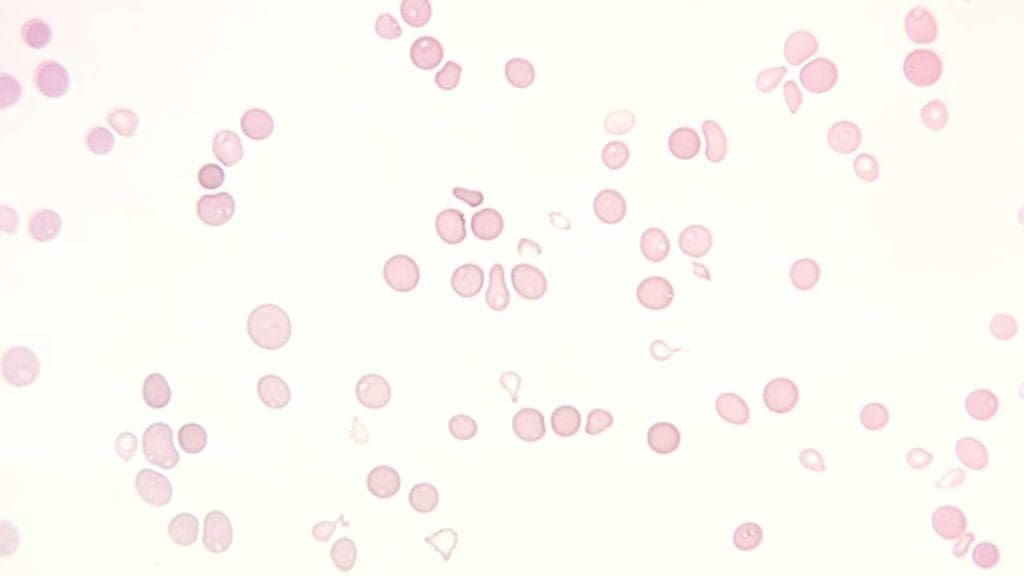
CBC measures the number of red blood cells, white blood cells, and platelets in the blood while peripheral blood smear allows for the morphological overview of the peripheral blood cells.
Expected Results: Pancytopenia, which is a decrease in all three types of blood cells.
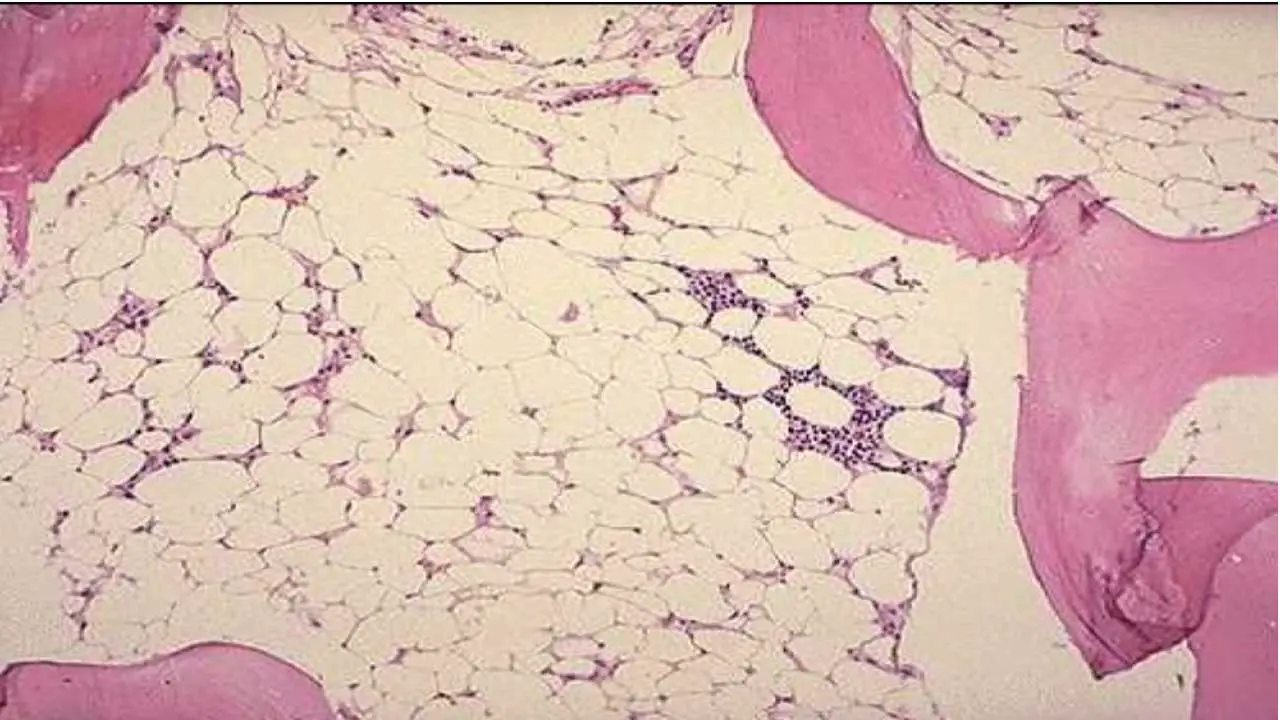
Bone Marrow Examination
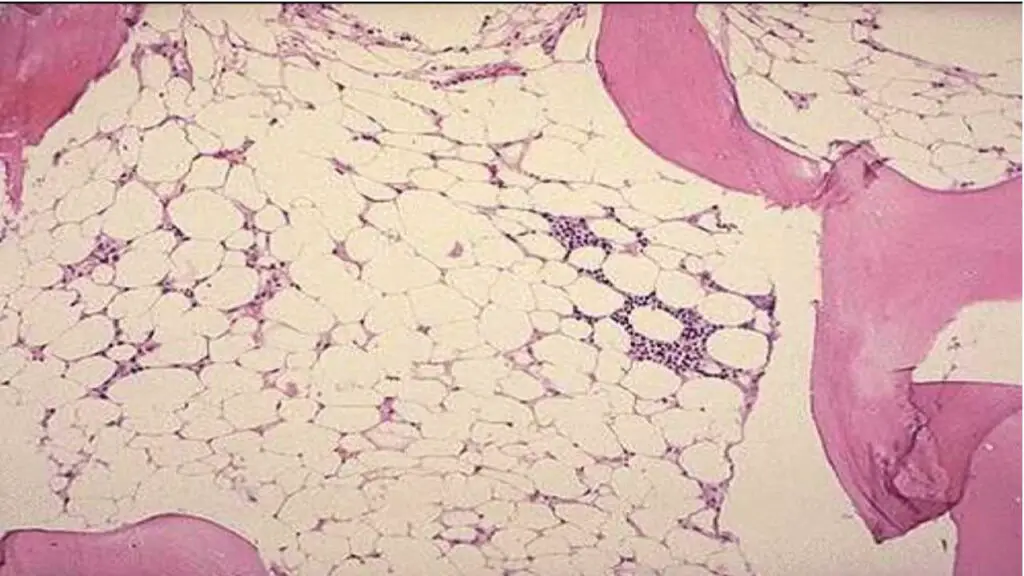
A bone marrow sample is examined under a microscope to assess the number and quality of blood cells.
Expected Results: Hypocellular bone marrow with a decreased number of blood cell precursors.
Genetic Testing
This test analyzes the DNA to identify mutations in the genes associated with Fanconi anemia.
Expected Results: Identification of specific mutations in one of the Fanconi anemia genes.
Additional Tests
- Flow Cytometry: This technique can be used to measure DNA damage and repair in cells.
- Fanconi Anemia Panel: A genetic test that can identify mutations in multiple Fanconi anemia genes simultaneously.
It’s important to note that a definitive diagnosis of Fanconi anemia often requires a combination of these tests. Early diagnosis is crucial for initiating appropriate treatment and management.
Treatment and Management
The treatment of Fanconi anemia (FA) primarily focuses on managing the complications associated with the disease, particularly bone marrow failure and an increased risk of cancer.
Hematopoietic Stem Cell Transplantation (HSCT)
HSCT is the only curative treatment for bone marrow failure in Fanconi anemia. It involves replacing the patient’s damaged bone marrow with healthy donor bone marrow. Healthy stem cells from a matched donor are transplanted into the patient’s body. These stem cells then repopulate the bone marrow and produce healthy blood cells.
Supportive Care
- Blood Transfusions: Regular blood transfusions are necessary to manage anemia and thrombocytopenia.
- Growth Factors: Growth factors, such as erythropoietin and granulocyte-colony stimulating factor (G-CSF), can help stimulate blood cell production.
- Antibiotics and Antifungal Agents: These medications are used to prevent and treat infections.
- Androgen Therapy: Androgens can stimulate red blood cell production and may help improve anemia.
Congenital Abnormalities
- Surgical Correction: To address physical abnormalities, such as heart defects or skeletal deformities.
- Specialized Therapies: To manage specific conditions, like kidney or gastrointestinal problems.
Cancer Surveillance and Treatment
Patients with Fanconi anemia need regular check-ups to monitor for the development of cancer. Early detection of cancer is crucial for successful treatment. Standard cancer treatments, such as chemotherapy, radiation therapy, and surgery, are used to treat cancer in individuals with Fanconi anemia.
Genetic Counseling
Genetic counseling can help families understand the risks of passing on the Fanconi anemia gene to their children. Prenatal testing can be offered to couples with a family history of Fanconi anemia to determine if their unborn child has the condition.
Supportive Care for Other Complications
- Physical Therapy: To manage skeletal abnormalities and improve mobility.
- Occupational Therapy: To help with daily living activities.
- Psychological Support: To address the emotional and psychological impact of the disease.
It’s important to note that the specific treatment plan for an individual with Fanconi anemia will depend on the severity of their symptoms and their overall health. Early diagnosis and comprehensive management are essential to improve the quality of life and prognosis of individuals with Fanconi anemia.
Prognosis
The prognosis of Fanconi anemia (FA) has improved significantly in recent years due to advancements in medical care, particularly hematopoietic stem cell transplantation (HSCT). However, it remains a serious condition with a complex outlook.
Factors Affecting Prognosis
- Severity of Bone Marrow Failure: Individuals with severe bone marrow failure have a poorer prognosis.
- Development of Cancer: The risk of developing cancer, especially leukemia and solid tumors, significantly impacts the overall prognosis.
- Response to Treatment: The effectiveness of treatments, particularly HSCT, can influence the long-term outcome.
Overall Prognosis
- Improved Survival Rates: With advancements in medical care, survival rates have improved, especially for individuals who undergo successful HSCT.
- Increased Cancer Risk: Despite improved survival, individuals with Fanconi anemia remain at an increased risk of developing cancer throughout their lives.
- Quality of Life: While treatments can improve the quality of life, individuals with Fanconi anemia may experience ongoing health challenges and require lifelong medical care.
It’s important to note that the prognosis for each individual with Fanconi anemia can vary widely. Early diagnosis, aggressive treatment, and careful monitoring are essential for optimizing outcomes and improving the quality of life for individuals with this condition.
Frequently Asked Questions (FAQs)
What is the difference between Fanconi anemia and aplastic anemia?
Fanconi anemia and aplastic anemia are both conditions that cause the bone marrow to stop producing enough blood cells. However, Fanconi anemia is a rare, inherited genetic disorder that also causes birth defects and an increased risk of cancer, while aplastic anemia can be caused by various factors, including exposure to toxins or certain medications, and typically does not involve birth defects or an increased cancer risk.
What is the most common anomaly in Fanconi anemia?
The most common anomaly in Fanconi anemia is short stature.
At what age is Fanconi anemia diagnosed?
Fanconi anemia is typically diagnosed between the ages of 2 and 15 years old. However, it can be diagnosed earlier or later depending on the severity of symptoms.
What is the difference between Fanconi syndrome and Fanconi anemia?
Fanconi anemia is a rare, inherited genetic disorder that primarily affects the bone marrow. It causes bone marrow failure, leading to anemia, thrombocytopenia, and neutropenia. Individuals with Fanconi anemia are also at an increased risk of developing certain types of cancer.
Fanconi syndrome is a kidney disorder that affects how the kidneys reabsorb certain substances. This can lead to excessive loss of electrolytes and other important substances in the urine. Fanconi syndrome can be caused by various factors, including genetic conditions, medications, and certain toxins.
While both conditions share a similar name, they are distinct disorders with different causes, symptoms, and treatments.
How long can someone live with Fanconi anemia?
The lifespan of individuals with Fanconi anemia varies greatly depending on the severity of the condition and the effectiveness of treatment. While the average lifespan has historically been shorter, advancements in medical care, particularly hematopoietic stem cell transplantation (HSCT), have significantly improved survival rates.
Approximately 80% of people with Fanconi anemia live to adulthood. However, the risk of developing cancer, especially leukemia and solid tumors, remains a significant challenge.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Peake JD, Noguchi E. Fanconi anemia: current insights regarding epidemiology, cancer, and DNA repair. Hum Genet. 2022 Dec;141(12):1811-1836. doi: 10.1007/s00439-022-02462-9. Epub 2022 May 21. PMID: 35596788.
- Dufour C, Pierri F. Modern management of Fanconi anemia. Hematology Am Soc Hematol Educ Program. 2022 Dec 9;2022(1):649-657. doi: 10.1182/hematology.2022000393. PMID: 36485157; PMCID: PMC9821189.
- Soulier J. Fanconi anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:492-7. doi: 10.1182/asheducation-2011.1.492. PMID: 22160080.
- Green AM, Kupfer GM. Fanconi anemia. Hematol Oncol Clin North Am. 2009 Apr;23(2):193-214. doi: 10.1016/j.hoc.2009.01.008. PMID: 19327579; PMCID: PMC5912671.