TL;DR
Dengue Virus and Transmission ▾
- Dengue is a viral infection spread by mosquitoes, particularly Aedes mosquitoes.
- There are four serotypes of the dengue virus, and infection with one serotype provides only temporary immunity to that specific type.
Clinical Presentation ▾
- Dengue infection can range from asymptomatic to a debilitating illness.
- Classic dengue fever presents with a sudden high fever, severe headache, muscle and joint aches, nausea, vomiting, and a rash.
- Atypical presentations can include mild illness, febrile rash illness, or involvement of various organs.
Complications ▾
- Severe dengue, characterized by Dengue Hemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS), can occur in secondary infections.
- DHF is characterized by plasma leakage leading to bleeding tendencies and fluid accumulation.
- DSS is a life-threatening complication of DHF with shock due to severe plasma leakage and decreased blood volume.
- Other complications include organ involvement (liver, heart, brain, kidneys) and Hemophagocytic Lymphohistiocytosis (HLH), a rare but severe hyperinflammatory syndrome.
Diagnosis ▾
- Diagnosis relies on a combination of clinical presentation, travel history, and laboratory tests.
- Viral detection tests (RT-PCR) are preferred for early diagnosis, while serological tests can be helpful later in the infection.
Management ▾
- There is no specific antiviral medication for dengue.
- Treatment focuses on managing symptoms, preventing complications, and providing supportive care:
- Rest, hydration, and pain management for mild cases.
- Intravenous fluids, electrolyte replacement, and blood transfusions for severe cases.
- Supportive care is also crucial for HLH management, along with medications to suppress the overactive immune system and address the underlying cause (if identified).
*Click ▾ for more information
Introduction
Dengue infection is a mosquito-borne viral illness caused by the dengue virus. It’s a systemic infection, meaning it can affect multiple organ systems in the body. The severity of the infection can range from completely asymptomatic (no symptoms) to a debilitating illness with potentially life-threatening complications.
Early Descriptions and Geographic Spread (992 AD – 19th Century)
- The earliest documented evidence of a dengue-like illness dates back to 992 AD in China. Descriptions in a Chinese medical encyclopedia detail symptoms consistent with dengue fever. They described the disease as ‘water poison’ and noted it to be associated with flying insects.
- Fast forward to the 18th and 19th centuries, with the expansion of global trade and shipping, dengue virus likely hitched a ride on ships, reaching new territories.
- The first account of a dengue outbreak in the Western Hemisphere came in 1780, reported in Philadelphia. This highlights the ability of the virus to travel long distances.
Dengue Hemorrhagic Fever Emerges and Classification System (20th – 21st Century)
- The development of a distinct clinical entity known as Dengue Hemorrhagic Fever (DHF) is attributed to Cuban physician Thomas Lane Bancroft in 1906. Thomas Lane Bancroft suggested that the mosquito vector Aedes aegypti transmitted dengue, and the following year in 1907, Ashburn and Craig proved that dengue was caused by a virus.
- DENV-1 was first identified in 1943 by Japanese scientists.
- DENV-2 was identified in 1944 by Albert Sabin.
- The 20th century saw a dramatic increase in dengue cases globally. This is likely due to factors like unplanned urbanization, population growth, and limitations in mosquito control.
- In 2012, the World Health Organization (WHO) established the current dengue classification system. This standardized system categorizes dengue infection based on severity, aiding in diagnosis, prognosis, and treatment decisions.
- WHO has also classified dengue as the most important mosquito-borne viral disease in the world.
Global Burden and Epidemiology of Dengue Infection
Dengue infection has become a major public health concern globally, particularly in tropical and subtropical regions. WHO has reported a marked rise of annual dengue cases since the 1950’s.
Magnitude of the Problem
- Dengue is considered the most rapidly spreading and geographically widespread mosquito-borne viral disease worldwide.
- Estimates suggest an annual range of 100 to 400 million dengue infections globally, with approximately 50 million symptomatic cases, 500,000 cases of severe dengue and over 20,000 dengue-related deaths.
- It’s crucial to note that a vast majority of cases are asymptomatic or mild, leading to under-reporting.
Geographical Distribution
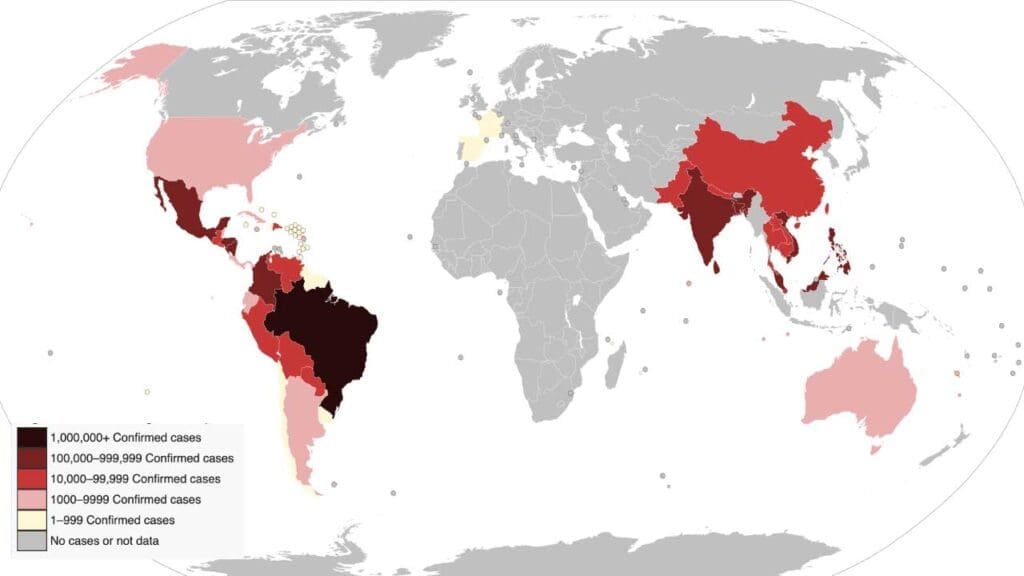
- Dengue is found across the tropics and subtropics, with a strong presence in Southeast Asia, the Western Pacific, the Americas, and Africa where the mosquito species Aedes aegypti and Aedes albopictus can be found.
- Over 3.6 billion people live in areas at risk of dengue infection.
- Urban and semi-urban areas are most affected due to factors like:
- Increased mosquito breeding grounds (stagnant water)
- High population density facilitating virus transmission
Epidemiological Trends
- The incidence of dengue has shown a dramatic rise in recent decades. Dengue epidemics are observed to be larger, more frequent and associated with more severe disease than they were in the past.
- WHO reported a ten-fold increase in reported cases between 2000 and 2019.
- Factors contributing to this rise include:
- Urbanization and population growth
- International travel and trade
- Climate change leading to expanded mosquito habitats
- Evolution and adaptation of the dengue virus
Dengue Virus: Serotypes and Antigenic Diversity
The dengue virus, belonging to the Flavivirus genus within the Flaviviridae family, is a single-stranded RNA virus. It’s responsible for causing dengue infection.
Serotypes and Classification
There are four well-recognized serotypes of the dengue virus, denoted as DEN-1, DEN-2, DEN-3, DEN-4, and a putative DEN-5. However, DEN-5 remains unconfirmed for its ability to cause human disease.
Antigenic Diversity
Despite sharing a significant portion of their genetic material (around 60- 80% homology), the dengue virus serotypes exhibit antigenic diversity. This means:
- The viral proteins on the surface of each serotype differ slightly.
- These differences impact how the immune system recognizes and responds to the virus.
- An individual infected with one serotype develops specific antibodies that can effectively neutralize that particular serotype (long-life protection).
- However, these antibodies might not be as effective against other serotypes due to antigenic diversity.
- This is a crucial factor in understanding the risk of severe dengue infection.
Viral Structure and Replication Cycle in the Human Body
Dengue Virus Structure
The dengue virus is a roughly spherical particle approximately 50 nanometers in diameter. It has a relatively simple structure but utilizes it effectively to invade and replicate within human cells. Here are the key components:
- Nucleocapsid: At the core lies the single-stranded RNA genome (~11kb), the blueprint for viral proteins. The 11-kb positive sense RNA genome encodes 3 structural proteins (capsid, prM, and E) and 7 nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5). It’s tightly associated with a protein shell called the capsid, which protects the RNA.
- Envelope: Surrounding the nucleocapsid is a lipid bilayer membrane, derived from the host cell during viral assembly. This envelope is studded with two key viral proteins:
- Envelope (E) protein: The most abundant protein on the surface, it mediates attachment to host cells and plays a role in viral fusion with the cell membrane.
- Membrane (M) protein: Anchors the E protein in the viral envelope and interacts with the host cell membrane during viral entry.
- The proteins present on the surface of the dengue immature virus are the E and prM. M protein is a cleaved derivative of prM and is found on the surface of a mature virus.
Dengue Virus Replication Cycle
The dengue virus, lacking its own machinery for replication, relies on hijacking the host cell’s resources. The dengue viral replication process begins when the virus attaches to a human skin cell.
- Attachment and Entry: Dengue virions bind to specific receptors on the surface of a susceptible human cell, such as dendritic cells or macrophages.
- Endocytosis: The virus-cell complex is engulfed by the host cell through a process called endocytosis, forming an internal vesicle. This pouch is called an endosome. A cell normally uses endosomes to take in large molecules and particles from outside the cell for nourishment. By hijacking this normal cell process, the dengue virus is able to enter a host cell.
- Uncoating: Once the virus has entered a host cell, the virus penetrates deeper into the cell while still inside the endosome. Researchers have learned that two conditions are needed for the dengue virus to exit the endosome: 1) The endosome must be deep inside the cell where the environment is acidic. 2) The endosomal membrane must gain a negative charge. Within the acidic environment of the endosome, the viral envelope fuses with the endosomal membrane, releasing the nucleocapsid into the cytoplasm of the cell.
- Viral RNA Translation: The viral RNA then hijacks the host cell’s machinery to replicate itself. The viral RNA serves as a messenger RNA (mRNA) for the host cell’s ribosomes. These ribosomes translate the viral RNA into a single long polyprotein.
- Polyprotein Processing: Cellular enzymes and viral proteases cleave the polyprotein into individual functional viral proteins, including enzymes needed for viral RNA replication.
- Viral RNA Replication: Using the viral RNA as a template, the viral enzymes synthesize a negative-strand RNA intermediate. This intermediate is then used to create new positive-strand RNA copies, identical to the original viral genome.
- Viral Assembly: New viral proteins and RNA genomes assemble at the endoplasmic reticulum (ER) of the host cell. The E protein interacts with the ER membrane, and the nucleocapsid is enveloped by a budded-off portion of the ER membrane. This step adds the viral envelope and protective outer layer.
- Maturation and Release: Newly formed viruses mature within the Golgi apparatus of the host cell. Finally, they are released from the infected cell by exocytosis, ready to infect new cells and continue the cycle.

Dengue Pathogenesis
Primary Dengue Infection
A primary dengue infection, the first encounter with the virus, typically results in a milder illness known as classic dengue fever. Here’s a breakdown of the key steps involved in the pathogenesis.
Cellular Targets
- The dengue virus primarily infects dendritic cells and macrophages, which are immune system cells crucial for antigen presentation and initiating the immune response.
- Dengue virus infects and replicates within dendritic cells, hindering their ability to effectively activate T-lymphocytes, a critical part of the adaptive immune system.
- Monocytes and macrophages can harbor the virus and contribute to the inflammatory response.
- Additionally, the virus can infect other cell types, including endothelial cells lining the blood vessels, leading to vascular leakage, a hallmark of severe dengue.
Viral Replication and Immune Activation
- Following infection, the virus replicates within these target cells, as described earlier.
- Viral replication triggers the host’s innate immune response, leading to the production of antiviral molecules like interferons and inflammatory mediators like cytokines.
Innate Immune Response: This is the body’s first line of defense, involving:
- Production of interferons and other inflammatory mediators to limit viral replication and activate immune cells.
- Activation of natural killer (NK) cells to directly kill virus-infected cells.
Adaptive Immune Response: This takes time to develop but provides long-lasting immunity. It involves:
- B-lymphocytes produce specific antibodies against the dengue virus serotype involved in the primary infection. These antibodies can effectively neutralize the virus and aid in its clearance.
- T-lymphocytes (cytotoxic T cells) recognizing and killing virus-infected cells.
Clearance of Virus and Clinical Manifestations
- In a primary infection, the combination of the innate and adaptive immune response usually leads to viral clearance within a week or two.
- The clinical manifestations of classic dengue fever are thought to be a result of the body’s immune response to the viral infection.
- Symptoms like fever, headache, muscle and joint pain (breakbone fever), and a rash can occur as the immune system works to eliminate the virus.
Important Considerations
- While the immune response effectively controls the primary infection, it might not completely eliminate all viral particles.
- These residual viral particles or immune complexes can linger in the bloodstream, potentially contributing to mild bleeding manifestations.
- The severity of classic dengue fever can vary depending on the infecting serotype and individual factors like age and overall health.
- Additionally, the specific immune response developed during a primary infection can influence the course of a subsequent infection with a different serotype which sets the stage for the potential development of severe dengue in secondary infections.
Secondary Dengue Infection and Antibody-Dependent Enhancement (ADE)
A secondary dengue infection occurs when an individual who has previously been infected with one serotype of the dengue virus encounters a different serotype. This seemingly simple scenario can lead to a more complex and potentially life-threatening situation due to a phenomenon called Antibody-Dependent Enhancement (ADE).
Antibody-Dependent Enhancement (ADE)
- During a primary infection, the body develops antibodies specific to the infecting serotype. These antibodies effectively neutralize the virus and aid in its clearance.
- However, in a secondary infection with a different serotype, pre-existing antibodies might bind to the new virus but fail to completely neutralize it. This incomplete binding can actually enhance the ability of the virus to enter immune cells, particularly monocytes and macrophages, through a process called Fc receptor-mediated endocytosis.
- Once inside these immune cells, the virus replicates efficiently, leading to a higher viral load compared to a primary infection.
Increased Immune Activation and Vascular Leakage
- The high viral load in secondary infection triggers a more vigorous immune response, leading to an excessive production of inflammatory mediators like cytokines and chemokines.
- This “cytokine storm” can damage the endothelial cells lining the blood vessels, leading to increased vascular permeability. This hallmark feature of DHF allows fluids to leak out of the blood vessels into the surrounding tissues, causing:
- Plasma leakage (reduction in blood volume)
- Edema (fluid accumulation in tissues)
- Organ dysfunction, if severe
Dengue Hemorrhagic Fever (DHF)
DHF is a severe form of dengue infection characterized by plasma leakage, leading to various clinical manifestations:
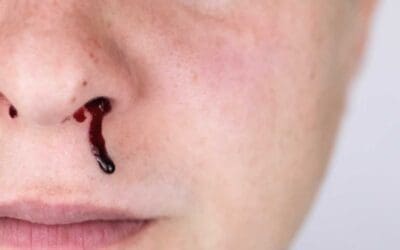
- Bleeding Tendencies: Plasma leakage can lead to a decrease in platelets (blood clotting cells) and various bleeding manifestations, ranging from easy bruising to severe bleeding from the nose, gums, or gastrointestinal tract.
- Fluid Accumulation: Leaked plasma accumulates in tissues, causing symptoms like swelling (edema) and potentially fluid buildup in the lungs (pleural effusion) or abdomen (ascites).
Dengue Shock Syndrome (DSS)
In some cases, DHF can progress to a life-threatening complication known as DSS. This occurs when severe plasma leakage leads to:
- Hypovolemia: A significant decrease in blood volume due to plasma leakage, leading to inadequate blood flow to vital organs.
- Shock: This can manifest as rapid weak pulse, cold and clammy skin, restlessness, and eventually organ failure if not promptly treated.
Risk Factors for Severe Dengue
- Secondary infection with a different serotype (particularly DEN-2 or DEN-3)
- Young children and adults are more susceptible to severe disease compared to older children.
- Underlying medical conditions like malnutrition or chronic diseases can increase the risk of complications.
Molecular Mechanisms in Dengue-induced Thrombocytopenia
Thrombocytopenia, a significant decrease in platelet count, is a common complication in dengue infection, particularly in severe cases like Dengue Hemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS). While the exact mechanisms remain under investigation, several potential pathways are thought to contribute to this phenomenon.
Decreased Platelet Production
- Bone Marrow Suppression by Dengue Virus
- The virus might directly infect hematopoietic progenitor cells in the bone marrow, the precursors that give rise to platelets. This can disrupt their normal maturation and release, leading to reduced platelet production.
- Infection of Stromal Cells
- Dengue virus might infect bone marrow stromal cells, which provide essential support for the growth and development of platelets. This infection can impair the function of these stromal cells, further hindering platelet production.
Increased Peripheral Platelet Destruction
- Platelet-Endothelial Cell and Platelet-Leukocyte Interactions
- Inflammatory mediators released during dengue infection can activate endothelial cells (lining of blood vessels) and leukocytes (white blood cells). These activated cells can interact with platelets, leading to their destruction.
- Platelet-Dengue Virus Interaction
- Dengue virus particles themselves might directly bind to platelets, triggering their activation and subsequent destruction by the immune system.
- Soluble Factors
- Dengue infection can lead to the production of various soluble factors like cytokines and chemokines. These factors can activate platelets and contribute to their destruction.
Additional Considerations
- Antibody-Dependent Enhancement (ADE): While primarily implicated in severe dengue pathogenesis, ADE may also play a role in platelet destruction.
- Pre-existing antibodies from a previous infection, in complex with a different serotype during a secondary infection, could target platelets for destruction by immune cells.
- Severity Dependence: The mechanisms contributing to thrombocytopenia may differ based on the severity of dengue infection.
- In mild cases, decreased production might be the primary factor, while increased destruction becomes more prominent in severe cases.
Importance of Understanding Mechanisms
Deciphering the molecular mechanisms behind dengue-induced thrombocytopenia is crucial for developing targeted therapies. Strategies to protect bone marrow from viral infection, inhibit platelet activation and destruction, or promote platelet production could potentially mitigate this complication and improve clinical outcomes.
Signs and Symptoms of Dengue Infection
Dengue infection can manifest in a wide range of presentations, from completely asymptomatic to a debilitating illness. Here’s a breakdown of the common signs and symptoms, including some atypical presentations:
Classic Dengue Fever
This is the most common presentation, particularly during a primary infection. Symptoms typically appear 4-10 days after exposure to the virus and may include:
- Sudden high fever (104°F/40°C or higher)
- Severe headache
- Pain behind the eyes
- Muscle and joint aches (often described as bone-breaking pain)
- Nausea and vomiting
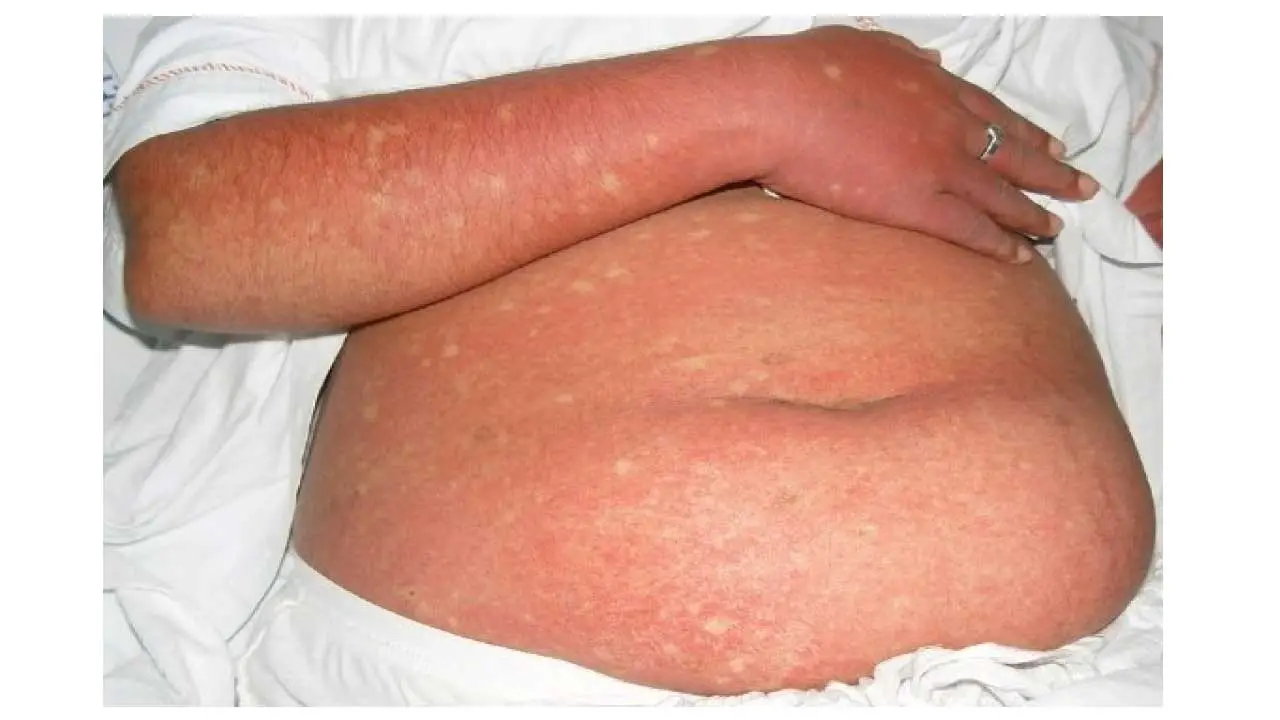
- Skin rash: This can appear 2-5 days after the fever onset, typically a red, flushed appearance on the face and upper body, sometimes followed by a more widespread itchy rash.
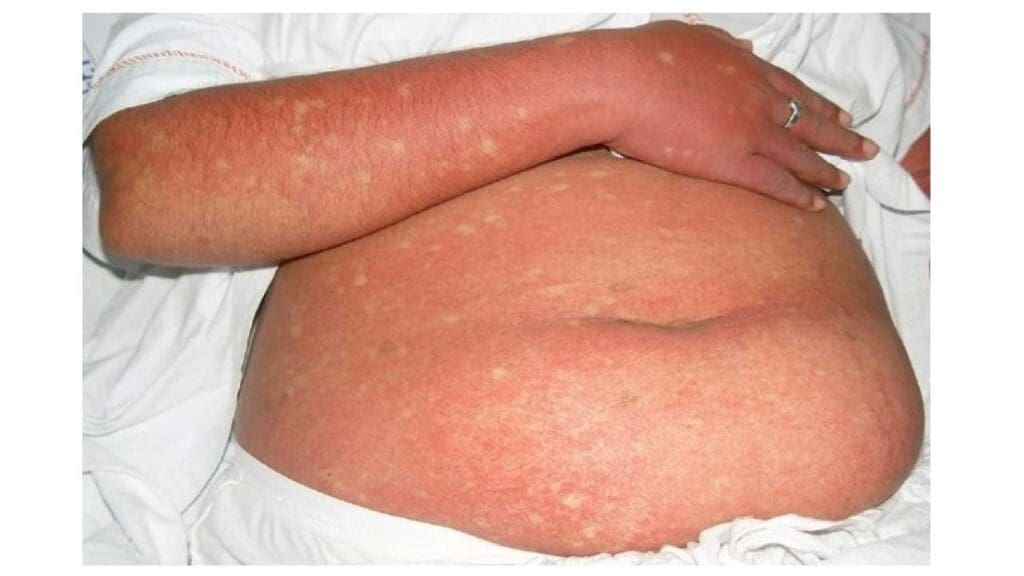
Atypical Presentations
Dengue infection can sometimes present in atypical ways, making diagnosis more challenging. Here are some examples:
- Mild Dengue: This may involve only a low-grade fever and some non-specific symptoms like malaise, fatigue, and mild muscle aches.
- Febrile Rash Illness: The rash might be the most prominent feature, with a less dramatic fever and other symptoms.
- Dengue with Warning Signs: This can occur in both primary and secondary infections. While not yet severe dengue, these warning signs indicate a potential risk of progression to DHF or DSS and require close monitoring:
- Severe abdominal pain
- Persistent vomiting (at least 3 times in 24 hours)
- Bleeding from the nose or gums
- Blood in vomit or stool
- Feeling tired, restless, or irritable
- Severe Dengue (DHF and DSS): These are characterized by plasma leakage and can manifest with additional symptoms like:
- Swelling (edema)
- Fluid accumulation in the lungs (pleural effusion) or abdomen (ascites)
- Rapid weak pulse
- Cold and clammy skin
- Restlessness and confusion (in severe cases)
Complications
While Dengue Hemorrhagic Fever (DHF) and Dengue Shock Syndrome (DSS) are the most widely recognized complications, other organ systems can also be affected. Additionally, Hemophagocytic Lymphohistiocytosis (HLH) can occur in rare instances.
Organ Involvement
Dengue virus can directly infect or indirectly damage various organs, leading to complications:
- Liver: Hepatitis (inflammation) can manifest with elevated liver enzymes, jaundice (yellowing of skin and eyes).
- Heart: Myocarditis (inflammation of the heart muscle) can occur, causing chest pain, shortness of breath, and abnormal heart rhythms.
- Brain: Encephalitis (inflammation of the brain) is a rare complication but can lead to seizures, altered consciousness, and coma.
- Kidneys: Acute kidney injury can develop, affecting urine output and waste removal from the body.
Hemophagocytic Lymphohistiocytosis (HLH)
This is a rare but potentially fatal complication of dengue infection, particularly in children. It’s a severe immune dysregulation characterized by:
- Excessive activation of immune cells: This leads to a cytokine storm, a massive release of inflammatory mediators that can damage tissues and organs.
- Hemophagocytosis: Immune cells (macrophages) start engulfing and destroying healthy blood cells, leading to cytopenias (low blood cell counts).
- Organ dysfunction: The cytokine storm and hemophagocytosis can damage vital organs like the liver, lungs, and central nervous system.
Laboratory Diagnosis of Dengue Infection
Dengue infection diagnosis relies on a combination of clinical presentation, travel history, and laboratory tests. Since symptoms in the early stages can be non-specific and overlap with other illnesses, laboratory confirmation plays a crucial role in guiding patient management.
Viral Detection Tests
- Real-time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR): This is the preferred method for early diagnosis (within the first 7 days of symptom onset). RT-PCR directly detects the presence of viral RNA (ribonucleic acid), the genetic material of the dengue virus, in the patient’s blood serum. A positive RT-PCR confirms an active dengue infection.
- Virus Isolation: This traditional method involves culturing patient serum in a susceptible cell line to isolate the live virus. While it can confirm the serotype (specific strain) of the infecting virus, it’s less sensitive and time-consuming compared to RT-PCR.
Serological Tests (Antibody Detection)
These tests detect antibodies produced by the immune system in response to the dengue virus infection. There are two main types of serological tests:
- Enzyme-Linked Immunosorbent Assay (ELISA): This commonly used test can identify IgM (Immunoglobulin M) and IgG (Immunoglobulin G) antibodies. IgM antibodies appear early in the infection (within the first few days) and indicate a recent or current infection. IgG antibodies develop later (around days 7-10) and persist for a longer duration. IgM antibodies typically indicate a recent primary infection, while IgG can be present in both primary and secondary infections. Detection of IgG antibodies suggests past dengue infection, even if asymptomatic.
- Rapid Diagnostic Tests (RDTs): These are point-of-care tests that provide quick results (usually within 15-30 minutes) using a finger prick blood sample. RDTs typically detect the presence of dengue NS1 (non-structural protein 1) antigen or IgM antibodies. While convenient for rapid diagnosis, RDTs may have limitations in sensitivity and specificity compared to ELISA.
Additional Laboratory Tests
- Full Blood Count (FBC): This provides information about blood cell counts, including platelets. Dengue infection can lead to thrombocytopenia (low platelet count), which is a crucial indicator for monitoring the risk of bleeding complications.
- Liver Function Tests (LFTs): These tests assess liver function and can reveal abnormalities suggestive of liver involvement in some dengue cases.
Choosing the Right Test
The choice of laboratory test depends on several factors, including:
- Stage of illness: Viral detection tests are preferred for early diagnosis, while serological tests might be more helpful in later stages.
- Availability of resources: RT-PCR is the gold standard but might not be readily available in all settings. RDTs offer a rapid alternative in resource-limited areas.
- Local epidemiology: Understanding the predominant serotypes circulating in a particular region can influence the interpretation of serological tests.
Differential Diagnosis
Dengue infection, particularly in its early stages, can mimic symptoms of other illnesses. Here’s a breakdown of some of the most common differential diagnoses to consider:
Viral Infections
- Chikungunya fever: This mosquito-borne viral illness can present with similar symptoms like fever, rash, and muscle aches. However, chikungunya typically causes more prominent joint pain and stiffness compared to dengue.
- Zika virus infection: Zika can cause a mild febrile illness with a rash, but it’s usually less severe than dengue. Additionally, Zika is a major concern for pregnant women due to its potential for birth defects.
- West Nile virus infection: This mosquito-borne virus can cause a range of illnesses, from mild fever to severe encephalitis (brain inflammation).
- Influenza: The flu typically presents with respiratory symptoms like cough, sore throat, and congestion, which are less prominent in dengue.
- Epstein-Barr virus (EBV) infection: EBV, the cause of mononucleosis, can cause fever, fatigue, and swollen lymph nodes, which can be confused with dengue.
Bacterial Infections
- Leptospirosis: This bacterial infection transmitted through contaminated water can cause fever, muscle pain, rash, and sometimes meningitis (inflammation of the meninges).
- Typhoid fever: This bacterial illness presents with fever, headache, malaise, and sometimes a characteristic rash.
- Rickettsial infections: These tick-borne or mite-borne bacterial infections can cause fever, rash, and headache, mimicking dengue.
Other Considerations
- Malaria: This parasitic infection transmitted by mosquitoes can cause fever, chills, sweating, and headache. Blood tests are essential to differentiate between malaria and dengue.
- Inflammatory conditions: Conditions like juvenile idiopathic arthritis or Kawasaki disease can present with fever, rash, and joint pain, requiring careful evaluation.
- Dengue with warning signs: Early stages of DHF or DSS can sometimes be confused with other causes of abdominal pain, vomiting, or bleeding.
Dengue Management and Treatment
There is no specific antiviral medication currently available to treat dengue infection. However, there are treatment strategies focused on managing symptoms, preventing complications, and providing supportive care.
Management of Mild Dengue Fever
- Rest: This allows the body to focus on fighting the infection.
- Hydration: Maintaining adequate fluid intake is crucial to prevent dehydration, especially with fever and vomiting. Oral rehydration solutions are often recommended.
- Pain Management: Over-the-counter pain relievers like acetaminophen (paracetamol) can help with fever and muscle aches. Important Note: Aspirin and ibuprofen should be avoided as they can increase the risk of bleeding.
- Monitoring: Close monitoring at home or in an outpatient setting is essential to track symptoms and identify any signs of worsening that might indicate a need for hospitalization.
Management of Severe Dengue (DHF and DSS)
- Hospitalization: Individuals with suspected DHF or DSS require immediate hospitalization for close monitoring and intensive supportive care.
- Fluid Management: This is the cornerstone of treatment. Intravenous fluids are administered to replace lost fluids due to plasma leakage and maintain blood pressure.
- Electrolyte Replacement: Electrolyte imbalances can occur due to fluid loss. Intravenous fluids can also help correct these imbalances.
- Blood Transfusions: In severe cases with significant bleeding, blood transfusions might be necessary to replace lost blood cells.
- Monitoring: Vital signs, urine output, and laboratory tests are closely monitored to assess response to treatment and identify potential complications.
General Prevention Strategies for Dengue
- Mosquito Control
- Eliminate stagnant water sources: Regularly empty and clean containers that can hold water, such as tires, flower pots, buckets, and clogged gutters.
- Cover water storage containers: Keep water storage containers tightly covered to prevent mosquitoes from laying eggs.
- Reduce outdoor breeding sites: Check around your home for any potential breeding grounds outside, like discarded items that can collect water.
- Personal Protection
- Use insect repellent: Apply EPA-registered insect repellents containing DEET, picaridin, IR3535, oil of lemon eucalyptus, or para-menthane-diol to exposed skin.
- Wear protective clothing: When outdoors, especially during peak mosquito biting times (dawn and dusk), wear long-sleeved shirts, long pants, and socks.
- Use mosquito nets: If sleeping in an area with no air conditioning or window screens, use mosquito nets to protect yourself while sleeping.
- Install window and door screens: Screens on windows and doors can prevent mosquitoes from entering your home.
- Community Awareness and Education
- Promote public awareness about dengue prevention strategies.
- Encourage community participation in mosquito control activities.
- Educate the public about the importance of early detection and seeking medical attention if symptoms of dengue develop.
Frequently Asked Questions (FAQs)
Can dengue spread from person to person?
No, dengue cannot spread directly from person to person. The dengue virus requires a specific mosquito vector to complete its life cycle and transmit the infection between humans. The primary mosquito vector for dengue is the Aedes aegypti mosquito.
Can dengue heal by itself?
Approximately 75-80% of dengue infections are mild and resolve on their own within a week or two.
Does dengue fever go down with paracetamol?
Yes, paracetamol (also known as acetaminophen) can be helpful in managing the fever and muscle aches associated with dengue fever.
Can we take antibiotics in dengue?
No, antibiotics are not effective against dengue fever because it is caused by a virus, not bacteria. Antibiotics specifically target bacterial infections by killing or stopping the growth of bacteria.
How many days do dengue rashes last?
The dengue rash typically lasts for 2-3 days, although it can persist for up to 5 days in some cases. The rash usually appears on Day 3 of the illness, following the initial fever and other symptoms. The rash might start as small, red, flat spots on the face and upper body. These can spread to the arms, legs, and trunk. The rash can have a flushed appearance initially and may evolve to become more maculopapular (raised bumps with a red base). The rash typically lasts for 2-3 days and then fades gradually. In some cases, it might linger for up to 5 days.
Why is dengue known as breakbone fever?
Dengue fever is known as breakbone fever due to the intensity of the muscle and joint pain experienced by many individuals infected with the virus. The dengue virus can directly infect muscle cells and connective tissues, leading to inflammation and pain. The body’s immune response to the virus can also contribute to muscle and joint pain through the release of inflammatory chemicals.
Are dengue and yellow fever the same?
No, dengue and yellow fever are not the same, although they are both mosquito-borne viral infections that can cause fever and other symptoms.
| Feature | Dengue Fever | Yellow Fever |
| Virus | Dengue virus (4 serotypes: DEN-1, DEN-2, DEN-3, DEN-4) | Yellow fever virus |
| Transmission | Aedes mosquitoes (primarily Aedes aegypti) | Aedes mosquitoes (various species) or Haemagogus mosquitoes in South America |
| Symptoms | Sudden high fever, severe headache, muscle and joint pain, nausea, vomiting, fatigue, rash (may not be present in all cases) | Sudden high fever, chills, headache, muscle aches, nausea, vomiting, weakness, jaundice (yellowing of the skin and eyes) in severe cases |
| Complications | Dengue Hemorrhagic Fever (DHF), Dengue Shock Syndrome (DSS) | Liver failure, kidney failure, bleeding problems, encephalitis (brain inflammation) |
| Geographic Distribution | Tropical and subtropical regions around the world | Sub-Saharan Africa and parts of South America |
| Vaccine | Dengue vaccine available in some countries, but not widely used and doesn’t offer complete protection against all serotypes | Highly effective and widely available vaccine |
| Prevention | Mosquito bite avoidance (insect repellents, protective clothing), eliminating mosquito breeding sites | Mosquito bite avoidance, vaccination |
Additional Points:
- Dengue infection can have a relatively mild presentation in some cases, whereas yellow fever typically causes a more severe illness.
- Dengue can lead to secondary infections, which can be more severe if a different serotype is involved. Yellow fever typically only causes one infection in a person’s lifetime.
- There is no specific antiviral medication for either dengue or yellow fever. Treatment focuses on managing symptoms and supportive care.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Rodenhuis-Zybert IA, Wilschut J, Smit JM. Dengue virus life cycle: viral and host factors modulating infectivity. Cell Mol Life Sci. 2010 Aug;67(16):2773-86. doi: 10.1007/s00018-010-0357-z. Epub 2010 Apr 6. PMID: 20372965.
- Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004 Jan 22;427(6972):313-9. doi: 10.1038/nature02165. PMID: 14737159.
- Whitehead S S, Blaney J E, Durbin A P, et al. Prospects for a dengue virus vaccine. Nature Reviews Microbiology, 2007, 5(7): 518-528.