Introduction
Blood film examination remains a cornerstone tool in diagnosing parasitic infections. These microscopic evaluations provide a window into the hidden world of parasites that can invade red blood cells (RBCs) and other components of the bloodstream. It provides a rapid, cost-effective, and readily available method for detecting a wide range of blood-borne parasites.
This practical approach plays a vital role in the diagnosis, management, and epidemiological surveillance of parasitic infections, including malaria, leishmaniasis, trypanosomiasis, filariasis (including loiasis), babesiosis, ehrlichiosis, and anaplasmosis. This laboratory protocol outlines the preparation and examination of blood films specifically tailored for detecting a range of common and geographically relevant parasites.
Principle
Two primary types of blood film preparations are employed in parasite diagnosis: thick and thin films. The principle behind thick blood film preparation lies in concentrating the parasite load within a larger volume of blood compared to a thin film. This significantly increases the sensitivity of parasite detection, especially for low parasite burdens.
- Thick Films: These prioritize sensitivity, aiming to concentrate parasites even when present in low numbers for example in malaria blood smear. A larger blood droplet is spread into a thicker layer. This concentrates white blood cells (WBCs) and parasites in a smaller area, allowing for faster screening. However, the lysing process can distort parasite morphology, hindering species identification.
- Thin Films (Peripheral Blood Smears): These prioritize detailed morphology for accurate parasite identification. A smaller blood droplet is spread into a thin layer, allowing individual cells to maintain their natural shape. This enables a clear visualization of parasite features crucial for species differentiation. However, thin films require a larger sample area to be examined, making them less sensitive for detecting low parasite burdens.
Applications in Specific Parasitic Diseases
- Malaria: Caused by Plasmodium species, malaria is a mosquito-borne parasitic disease affecting millions globally. Blood film examination remains the gold standard for malaria diagnosis. Malaria blood smear thick films are used for rapid screening due to their high sensitivity for detecting Plasmodium parasites. Thin films then allow for species identification and parasite quantification (parasitemia).
- Leishmaniasis: Transmitted by sandflies, Leishmania parasites can cause a spectrum of clinical presentations, including cutaneous, mucocutaneous, and visceral forms. Thin blood films can be used to detect amastigotes (intracellular) of Leishmania parasites within peripheral blood monocytes. However, bone marrow aspiration or splenic aspirate smears might be needed for a definitive diagnosis in some cases.
- Trypanosomiasis: Trypanosoma parasites, transmitted by tsetse flies in Africa and kissing bugs in Latin America, cause human African trypanosomiasis (sleeping sickness) and Chagas disease, respectively. Thick films can be employed to detect trypanosomes (Trypanosoma species) but Trypanosoma cruzi is a delicate parasite and can be easily damaged during the spreading process for thick blood films. However, in African trypanosomiasis, parasite numbers are often low, necessitating concentration techniques like buffy coat examination or minicolumn centrifugation alongside blood films.
- Filariasis and Loiasis: Caused by various filarial nematodes transmitted by mosquitoes, these infections can lead to lymphatic filariasis and loiasis (characterized by Calabar swellings). Microscopic examination of blood films is primarily used for loiasis diagnosis. Thick blood films are drawn at specific times (typically at night) to detect the characteristic microfilariae of Loa loa. Nighttime blood draws are crucial as these parasites exhibit nocturnal periodicity..
- Babesiosis, Ehrlichiosis, and Anaplasmosis: These tick-borne diseases caused by Babesia, Ehrlichia, and Anaplasma species, respectively, can mimic malaria with flu-like symptoms and RBC infection. Thin blood films are the primary diagnostic tool for these tick-borne infections. They allow visualization of intraerythrocytic inclusions containing these organisms (Babesia) or morulae (Ehrlichia and Anaplasma) within red blood cells.
Materials
- Capillary blood (studies have shown that for some parasites, particularly malaria parasites (Plasmodium species), capillary blood might have a higher concentration compared to venous blood)
- Glass slide and spreader
- 3% Giemsa stain made from Giemsa stock solution and Giemsa buffer solution
- Phosphate buffer (pH 6.8)
- Coplin jar or staining tray
- Drying rack
- DPX and cover slip
Method
- Make sure the glass slide and spreader are clean using the lens paper.
- Place 1 drop of capillary blood in the center of the slide.
- Gently spread the blood drop in a circular motion using the corner of a spreader. Aim for a smear roughly the size of a dime (around 1-2 cm in diameter) and with a moderate thickness. It shouldn’t be so thick that you can’t see light passing through it when held up to a newspaper.
- Let the smears dry completely on a flat surface, making sure to keep them away from dust and insects. This is crucial because smears that aren’t dry enough (especially thick ones) or those made with blood containing anticoagulant are more likely to detach from the slide during staining. Drying can take several hours at room temperature with a minimum of 30 minutes depending on the room temperature.
- Prepare the 3% Giemsa stain solution according to the manufacturer’s instructions.
- Fill a staining dish or Coplin jar with the 3% Giemsa stain solution.
- Using staining racks, carefully place the blood smears (thick and/or thin films) into the staining dish, ensuring they are completely submerged in the stain solution.
- Incubate the slides in the Giemsa stain solution for the recommended time (approximately 30 to 45 minutes) depending on the manufacturer’s instructions.
- After the staining time is complete, carefully remove the slides from the stain solution and rinse them gently in a buffer solution (usually phosphate buffer, pH 6.8) to remove excess stain.
- Following the buffer rinse, give the slides a final rinse with distilled water to remove any residual buffer or stain.
- Stand the slides upright on a staining rack or absorbent paper to allow them to air dry completely. This can take 15-30 minutes. Avoid using heat lamps or direct sunlight, as this can damage the cells.
- Once completely dry, mount the smear with DPX and cover with a cover slip. The stained blood smears are ready for examination under a microscope using oil immersion lens for optimal visualization of cells and potential parasites.
Important Note: Thick blood smears should not be fixed with methanol or heat, as this can damage the parasites and make them difficult to see. However, if staining the smears will be delayed, you can briefly dip the thick smear in water to lyse (break open) the red blood cells (RBCs). This allows for better visualization of parasites during staining.
Interpretation
Information from the blood smear includes:
- Parasite Morphology: Shape, size, and internal structures of the parasite can aid in species identification.
- Parasite Stage: Identifying different life stages of the parasite (e.g., ring forms, trophozoites, gametocytes in malaria blood smear) can provide insights into the course of infection.
- Parasite Burden: The number of infected red blood cells compared to healthy ones (parasitemia) in thin films helps assess disease severity and guide treatment decisions.
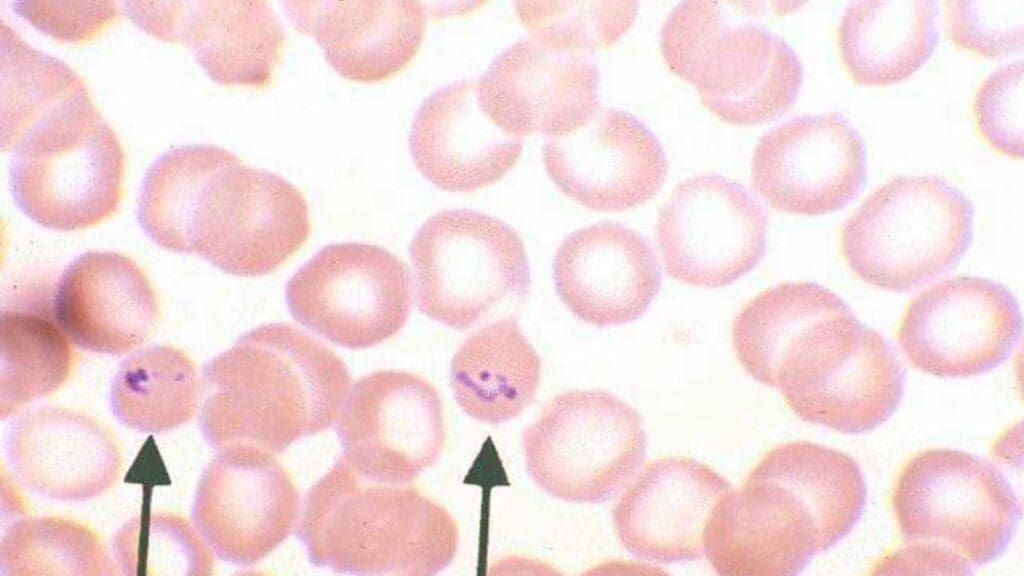
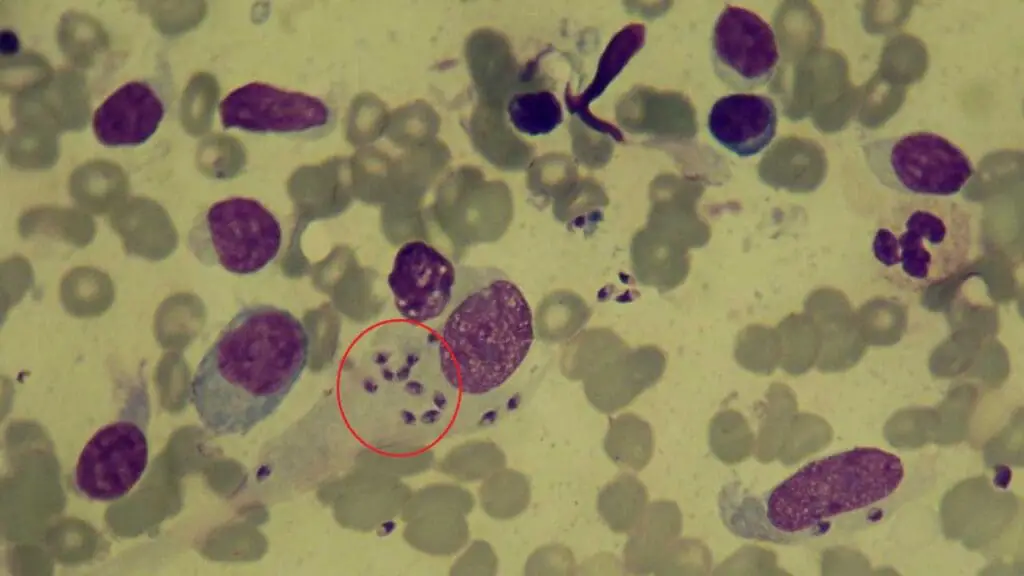
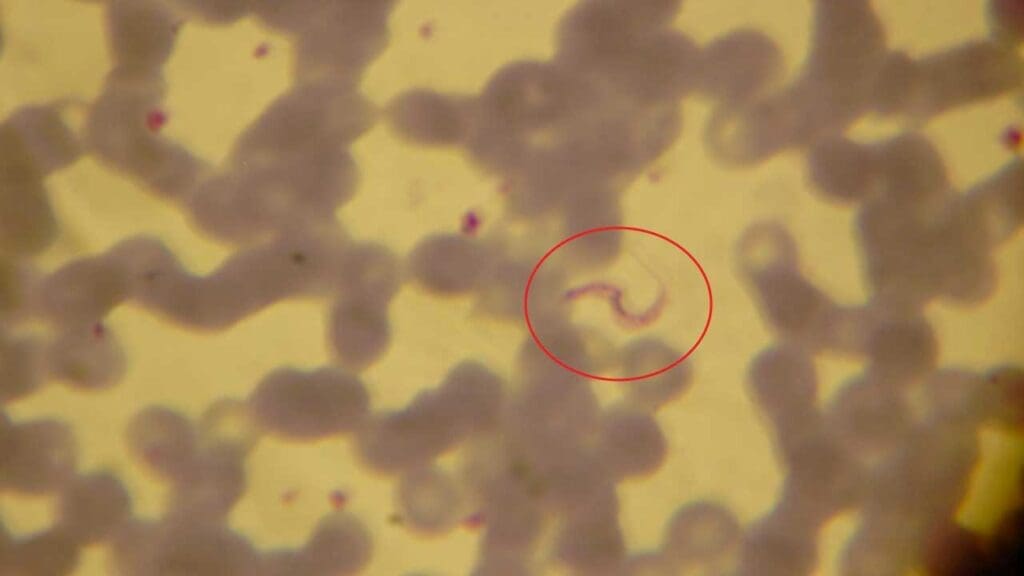
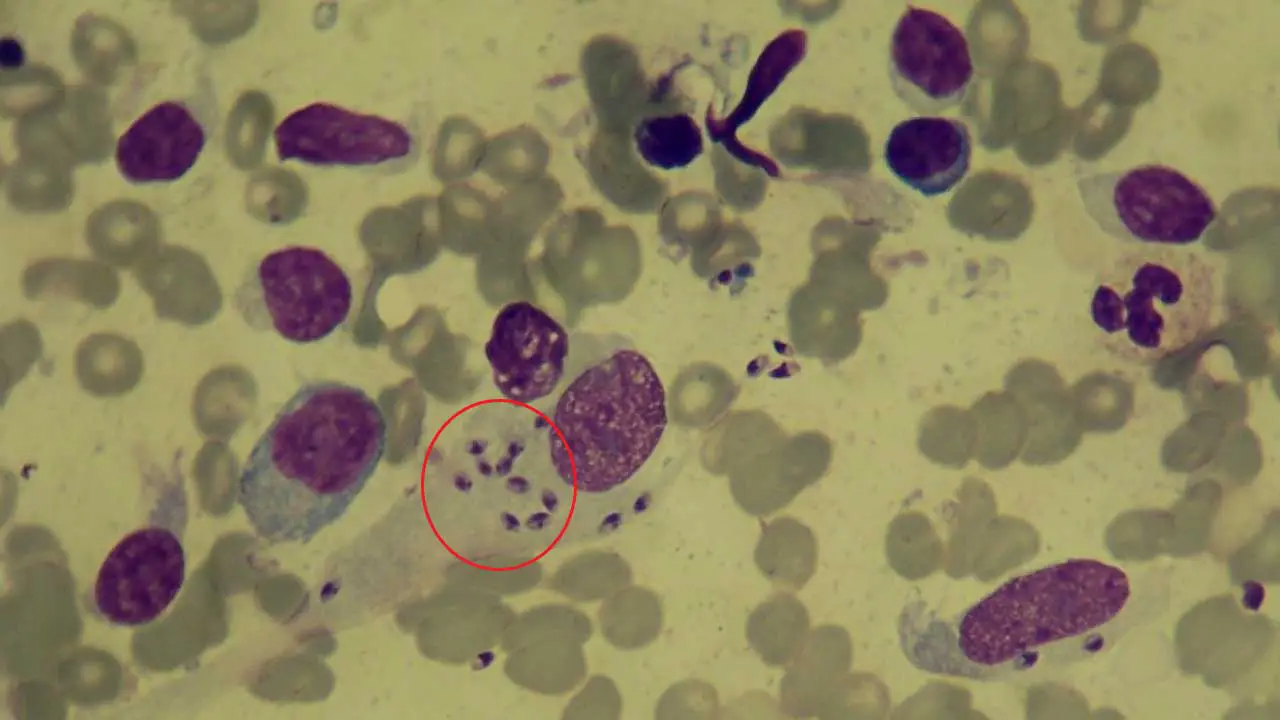
Limitations of Thick Blood Films
While blood film examination is a powerful diagnostic tool, it has limitations.
- Parasite Burden: Certain parasites might be present in very low numbers, especially during the early stages of infection, potentially leading to negative results despite the presence of disease.
- Specificity: In some cases, differentiating between parasite species based solely on morphology can be challenging. Additional diagnostic tests like serological assays or molecular techniques might be necessary for definitive identification.
- Technical Expertise: Accurate diagnosis relies heavily on the expertise of the microscopist in recognizing parasite morphology and differentiating them from artifacts or other blood components.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.