TL;DR
Methemoglobinemia is a blood disorder where the iron in hemoglobin is oxidized from its normal ferrous state (Fe2+) to the ferric state (Fe3+). This abnormal hemoglobin, called methemoglobin (MetHb), can’t carry oxygen.
- Pathophysiology ▾: It causes a functional anemia by directly reducing oxygen-carrying capacity. It also causes a leftward shift of the oxyhemoglobin dissociation curve, meaning the remaining normal hemoglobin holds onto oxygen more tightly, preventing its release to tissues.
- Causes ▾:
- Congenital (Inherited): Caused by genetic defects, such as a deficiency in the NADH-cytochrome b5 reductase enzyme or a structural mutation in the hemoglobin itself (Hemoglobin M disease).
- Acquired (Most Common): Triggered by exposure to oxidizing agents like certain drugs (local anesthetics such as benzocaine, dapsone, nitrates) or toxins (nitrites in well water).
- Diagnosis ▾:
- Pulse Oximetry
- Co-oximetry (Gold standard)
- Saturation Gap
- Treatment ▾: Methylene blue but contraindicated in G6PD deficiency.
*Click ▾ for more information
What is methemoglobinemia?
Methemoglobinemia is a hematologic condition where the iron in hemoglobin is oxidized from its normal ferrous state (Fe2+) to the ferric state (Fe3+).
In this state, known as methemoglobin (MetHb), the molecule can no longer bind and transport oxygen effectively. This leads to a type of functional anemia and tissue hypoxia, as the body’s cells are starved of oxygen despite normal amounts of hemoglobin being present.
Understanding this condition is crucial for medical students because its signs and symptoms are directly related to the percentage of methemoglobin in the blood, and prompt, specific treatment can be life-saving.
Normal Physiology of Hemoglobin
Hemoglobin’s primary function is to transport oxygen from the lungs to the tissues. This process relies on a reversible binding mechanism governed by the molecule’s structure and its environment.
The hemoglobin is a complex protein in red blood cells composed of four globin chains, each with a central heme group. At the core of each heme group lies an iron atom in the ferrous (Fe2+) state.
This ferrous iron is what reversibly binds to oxygen molecules, allowing a single hemoglobin molecule to transport up to four molecules of oxygen.
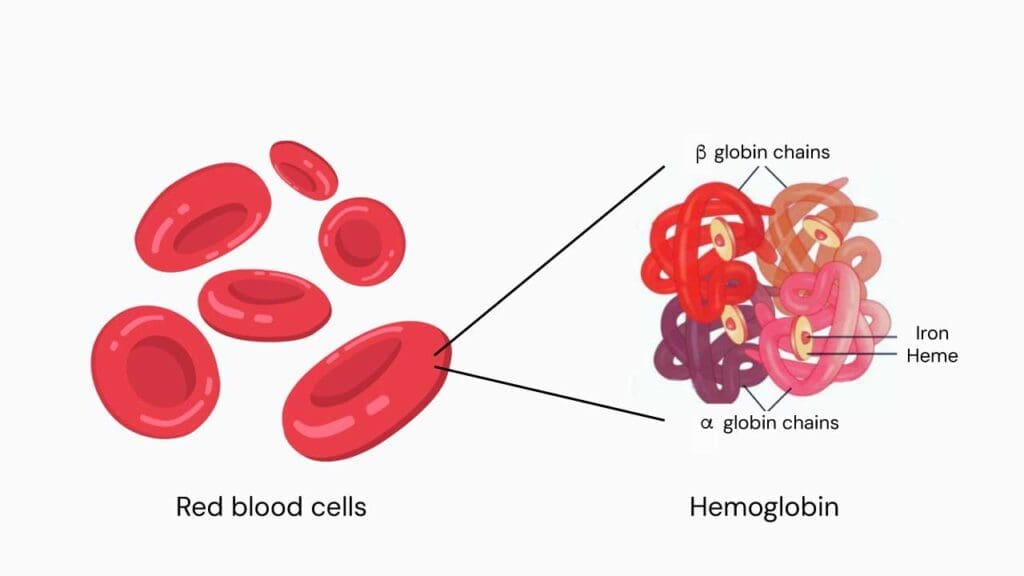
Oxygen Binding in the Lungs
In the lungs, where the partial pressure of oxygen (pO2) is high, oxygen molecules diffuse into the red blood cells.
Each of the four heme groups in a hemoglobin molecule contains a ferrous (Fe2+) iron atom. The binding of the first oxygen molecule causes a conformational change in the hemoglobin, shifting it from a low-affinity “Tense” (T) state to a high-affinity “Relaxed” (R) state.
This change makes it easier for the other three oxygen molecules to bind, a phenomenon known as positive cooperativity.
Oxygen Release in the Tissues
When the red blood cell reaches the tissues, the conditions are different. The pO2 is lower, and the environment is more acidic due to higher levels of carbon dioxide (CO2) and protons (H+).
These factors, collectively known as the Bohr effect, along with the presence of 2,3-bisphosphoglycerate (2,3-BPG), cause hemoglobin to revert to its low-affinity T state.
This conformational change weakens the bond between hemoglobin and oxygen, prompting the release of oxygen molecules into the surrounding tissues where they are needed for cellular metabolism. This ensures efficient oxygen delivery precisely where it is required.
Methemoglobin Reductase Pathway
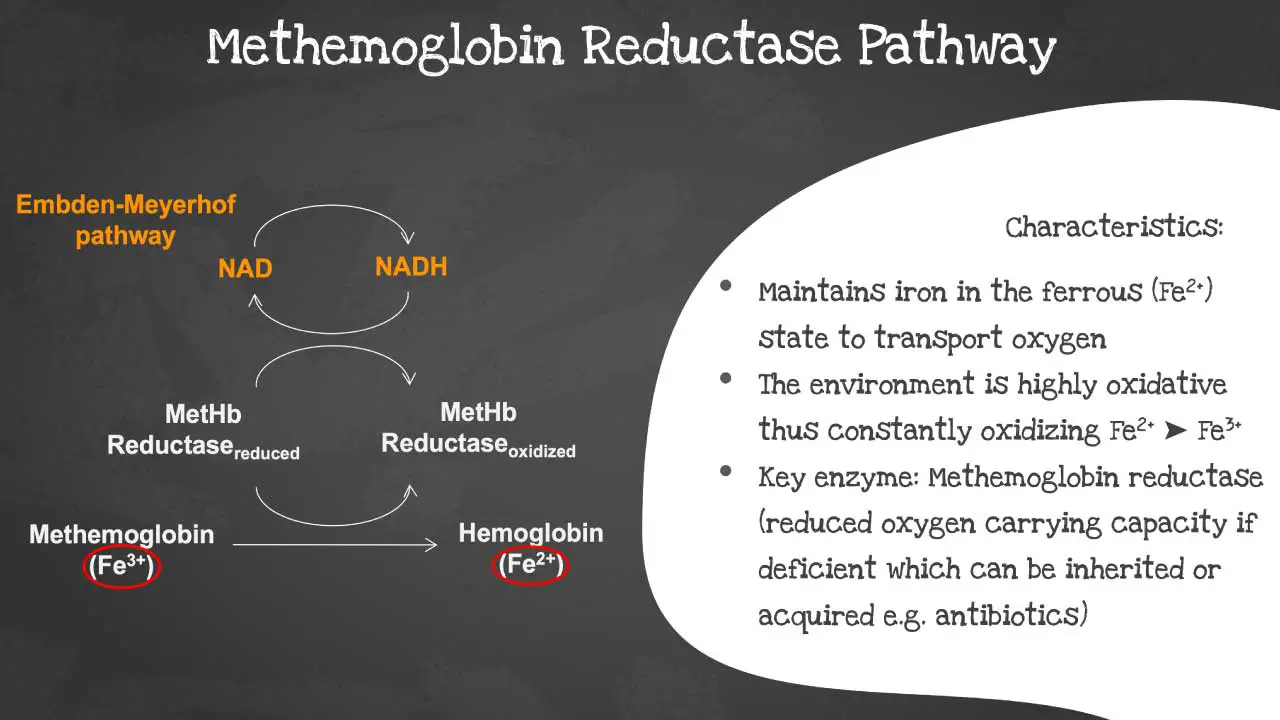
Under normal physiological conditions, a small amount of hemoglobin is spontaneously oxidized to methemoglobin (Fe2+), where the iron is in the ferric (Fe3+) state.
To prevent this from accumulating, a critical enzyme system within the red blood cell, the NADH-cytochrome b5 reductase pathway, constantly works to reduce methemoglobin back to functional hemoglobin. This pathway uses NADH, a product of glycolysis (the Embden-Meyerhof pathway), as an electron donor.
The electron is passed to a protein called cytochrome b5, and then cytochrome b5 reductase catalyzes the final step of transferring the electron to the ferric iron of methemoglobin, restoring it to its ferrous state (Fe2+). This process is highly efficient and maintains methemoglobin levels at less than 1% of total hemoglobin in healthy individuals.
Causes of Methemoglobinemia
Methemoglobinemia can be caused by both genetic and environmental factors. The underlying issue is that the mechanisms for reducing methemoglobin back to its functional form are overwhelmed, leading to a build-up of the non-oxygen-carrying form of hemoglobin.
Congenital Causes
These are genetic conditions that affect the body’s ability to reduce methemoglobin.
Hereditary Methemoglobinemia (NADH-cytochrome b5 reductase deficiency)
This is the most common form of congenital methemoglobinemia.
- Type I: A less severe form where the enzyme deficiency is limited to red blood cells. Symptoms are generally mild and often include only a bluish discoloration of the skin (cyanosis). Patients typically have a normal life expectancy.
- Type II: A much rarer and more severe form where the enzyme deficiency affects all cells in the body. In addition to cyanosis, patients experience severe neurological issues, developmental delays, and often do not survive past early childhood.
Hemoglobin M Disease
This is a rare, autosomal dominant condition resulting from a mutation in the globin gene. The mutation creates a structurally unstable hemoglobin variant, known as Hemoglobin M (HbM), that is more susceptible to oxidation and resistant to reduction by the normal enzyme pathways. Patients with this condition are born with chronic cyanosis.
Acquired Causes
This is the most common form of methemoglobinemia, resulting from exposure to certain drugs, chemicals, or toxins. These substances act as oxidizing agents that convert the iron in hemoglobin from the ferrous (Fe2+) to the ferric (Fe3+) state faster than the body’s natural reduction pathways can keep up.
Drugs
Numerous medications can act as potent oxidizing agents. The most common culprits include:
- Local anesthetics: Benzocaine, prilocaine, lidocaine, and dapsone are well-known triggers, particularly in topical preparations or when used in high doses.
- Antibiotics: Dapsone (used for leprosy and Pneumocystis pneumonia), sulfonamides, and trimethoprim.
- Urinary tract analgesics: Phenazopyridine.
- Nitrates and Nitrites: Nitroglycerin (a common heart medication), sodium nitroprusside, and amyl nitrite (a recreational drug).
- Antimalarials: Primaquine and chloroquine.
- Chemotherapy agents: Rasburicase.
Toxins and Chemicals
Exposure to environmental toxins and chemicals can also lead to methemoglobinemia, such as:
- Nitrites in well water: This is a classic cause of “blue baby syndrome” in infants, who are highly susceptible to methemoglobinemia.
- Industrial chemicals: Aniline dyes and nitrobenzene.
- Fertilizers and pesticides: Certain agricultural chemicals can contaminate food and water sources.
- Oxides of Nitrogen: Exposure to these can occur during industrial processes or in cases of smoke inhalation.
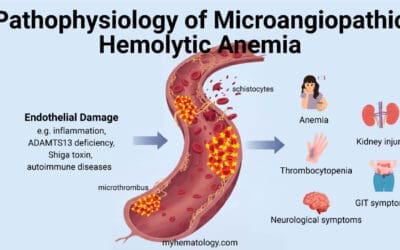
Pathophysiology of Methemoglobinemia
The pathophysiology of methemoglobinemia is a two-fold process that impairs oxygen transport and delivery.
Impaired Oxygen Binding
The central issue is the oxidation of the iron atom in the heme group from the ferrous (Fe2+) to the ferric (Fe3+) state.
This transformation, caused by either an overwhelming exposure to oxidizing agents or a genetic defect, renders that heme group incapable of reversibly binding oxygen. This directly reduces the overall oxygen-carrying capacity of the blood, leading to a state of functional anemia.
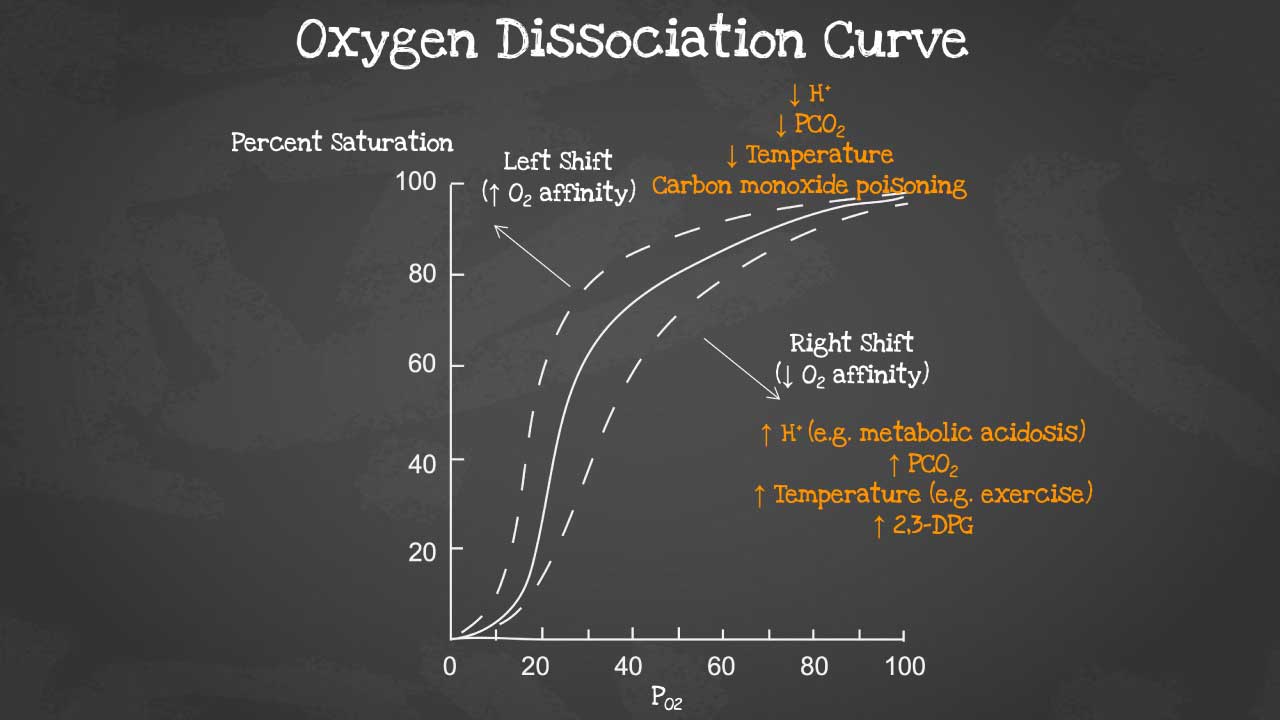
Leftward Shift of the Oxyhemoglobin Dissociation Curve
The second, and often more dangerous, effect is the impact on the remaining, un-oxidized hemoglobin molecules.
The presence of methemoglobin causes an allosteric change that forces the functional hemoglobin to stay in a high-affinity state. This significantly increases the affinity of the remaining hemoglobin for oxygen.
As a result, the oxyhemoglobin dissociation curve shifts to the left, meaning that while oxygen can still be picked up in the lungs, it is not released efficiently to the peripheral tissues where it’s desperately needed.
This is the primary reason for severe tissue hypoxia, even when the patient’s methemoglobin levels are only moderately elevated.
The combined effect of reduced oxygen-carrying capacity and impaired oxygen unloading in the tissues creates a vicious cycle that can quickly lead to life-threatening complications.
Signs and Symptoms of Methemoglobinemia
Methemoglobinemia symptoms can range from a subtle physical sign to life-threatening complications. A patient’s age and pre-existing health conditions can also influence how they present. Clinical signs are directly related to the percentage of methemoglobin in the blood.
MetHb Levels and Corresponding Symptoms
- <10−15%: Often asymptomatic, but the patient may have a slight grayish or dusky appearance.
- 15−30%: Cyanosis (bluish discoloration of the skin and mucous membranes), often described as “chocolate-colored blood” because the oxidized methemoglobin cannot bind oxygen and is therefore dark.
- 30−50%: Headache, dizziness, fatigue, and dyspnea (shortness of breath).
- 50−70%: This is a severe, life-threatening range. Symptoms include altered mental status, cardiac arrhythmias, and metabolic acidosis. The patient may also experience seizures and central nervous system depression.
- >70%: Levels this high are often fatal, leading to severe tissue hypoxia, circulatory collapse, and coma..
Laboratory Investigations and Diagnosis of Methemoglobinemia
Pulse Oximetry
This non-invasive method is often the first tool used to assess a patient’s oxygenation. However, in methemoglobinemia, it provides an inaccurate reading.
Standard pulse oximeters use two specific wavelengths of light to measure the ratio of oxyhemoglobin to deoxyhemoglobin.
Methemoglobin absorbs light similarly at both of these wavelengths, causing the pulse oximeter reading (SpO2) to trend toward and plateau at a value around 85%, regardless of the true oxygen saturation.
Co-oximetry
This is the gold standard for diagnosing methemoglobinemia. Unlike standard pulse oximeters, a co-oximeter uses multiple wavelengths of light (4 or more) to accurately measure the concentrations of all the different hemoglobin species in the blood, including oxyhemoglobin, deoxyhemoglobin, carboxyhemoglobin, and most importantly, methemoglobin (MetHb).
This provides a precise percentage of methemoglobin, which is crucial for determining the severity of the condition and guiding treatment.
Arterial Blood Gas (ABG)
While an ABG is not specific for methemoglobinemia, it can provide a critical clue.
In a patient with significant methemoglobinemia, the ABG may show a normal or even high partial pressure of oxygen (PaO2), which represents the oxygen dissolved in the blood. However, the calculated oxygen saturation (SaO2) may be normal, while the patient is clearly cyanotic and the pulse oximeter shows a low reading.
This discrepancy between the pulse oximeter reading and the ABG’s PaO2 and SaO2 is known as a “saturation gap,” which should immediately raise suspicion.
Ancillary Tests
A complete blood count (CBC) may be ordered to rule out other causes of cyanosis or to assess for co-existing anemia.
A toxicology screen may be helpful if an acquired cause (e.g., drug overdose, chemical exposure) is suspected.
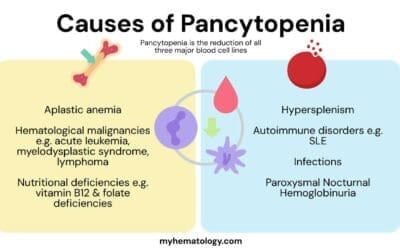
Differential Diagnosis of Methemoglobinemia
The presentation of cyanosis can be a red flag for many different conditions. It is important to consider other causes to avoid misdiagnosis, as the treatments for each are very different. The most important distinguishing factors are the patient’s history, the response to supplemental oxygen, and specific lab results, particularly co-oximetry.
Sulfhemoglobinemia
This rare condition is caused by the irreversible oxidation of hemoglobin, often due to exposure to sulfonamides or phenacetin. Like methemoglobinemia, it can cause cyanosis that is unresponsive to oxygen.
A key differentiating feature is that the blood may have a greenish tinge, and crucially, it is not detected by co-oximetry as methemoglobin and does not respond to methylene blue treatment.
Cardiopulmonary Disease
The most common causes of central cyanosis are cardiovascular or pulmonary in origin. Conditions such as congenital heart disease, severe heart failure, or pulmonary emboli can lead to poor oxygenation.
Unlike methemoglobinemia, these conditions will typically show a low on an arterial blood gas, and the cyanosis and hypoxemia may improve with supplemental oxygen.
Carboxyhemoglobinemia (Carbon Monoxide Poisoning)
Carbon monoxide binds to hemoglobin with an affinity much higher than oxygen, leading to impaired oxygen transport. Patients may present with nonspecific symptoms like headache and dizziness, but they often have a cherry-red complexion rather than cyanosis.
The pulse oximeter is notoriously unreliable, giving a falsely high reading. The diagnosis is confirmed by co-oximetry, which will show an elevated carboxyhemoglobin level.
Argyria
A rare, cosmetic condition resulting from prolonged ingestion or exposure to silver compounds. It causes a permanent, diffuse blue-gray discoloration of the skin and mucous membranes.
Unlike methemoglobinemia, it is not a systemic disease and does not cause hypoxia or other acute symptoms.
Treatment and Management of Methemoglobinemia
The primary goal of treating methemoglobinemia is to restore the oxygen-carrying capacity of the blood by reducing methemoglobin back to functional hemoglobin.
Treatment is generally indicated for patients with methemoglobin levels greater than 20-30% or for symptomatic patients, regardless of the level.
Immediate Steps
- Discontinue the offending agent: The first and most crucial step is to immediately identify and stop any exposure to the drug or toxin causing the condition.
- Administer supplemental oxygen: While oxygen alone does not directly reduce methemoglobin, it can increase the small amount of dissolved oxygen in the plasma (), which can provide some symptomatic relief while definitive treatment is initiated.
Specific Therapy: Methylene Blue
Methylene blue is the most effective and widely used antidote. It acts as an artificial electron carrier to accelerate a secondary reduction pathway.
In the presence of the enzyme NADPH-methemoglobin reductase (also known as NADPH diaphorase) and its coenzyme NADPH, methylene blue is reduced to leuco-methylene blue. Leuco-methylene blue then directly reduces the ferric iron (Fe3+) of methemoglobin back to the ferrous state (Fe2+), restoring its ability to bind oxygen.
The standard dose is 1−2 mg/kg of a 1% solution administered intravenously over a period of 5 minutes. The patient’s response should be monitored closely, and the dose may be repeated after an hour if the methemoglobin level has not decreased sufficiently or if symptoms persist.
Special Considerations
- Contraindications: Methylene blue should not be used in patients with G6PD (glucose-6-phosphate dehydrogenase) deficiency. In these individuals, the NADPH-methemoglobin reductase pathway is impaired due to a lack of NADPH. Administering methylene blue can lead to a severe, life-threatening hemolytic crisis by causing a massive oxidative stress on their red blood cells. It is also ineffective in treating sulfhemoglobinemia.
- Alternative Therapies: For patients with G6PD deficiency, or for those who do not respond to methylene blue, other treatments may be considered. Ascorbic acid (Vitamin C) can act as a slow, non-enzymatic reducing agent and may be used in mild cases or as an adjunct therapy. In severe, life-threatening cases unresponsive to all other measures, exchange transfusion may be a life-saving option to rapidly remove the methemoglobin-containing red blood cells.
Special Populations
Infants
Infants are particularly susceptible to methemoglobinemia due to a combination of physiological factors. They have a lower activity of the primary methemoglobin-reducing enzyme, NADH-cytochrome b5 reductase, and have higher levels of fetal hemoglobin, which is more easily oxidized to methemoglobin.
This makes them highly vulnerable to acquired causes of methemoglobinemia, such as contamination of well water with nitrites (a condition known as “blue baby syndrome”), which can cause severe illness at very low nitrite exposures compared to adults.
Patients with G6PD Deficiency
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is a common genetic disorder, particularly in individuals of African, Asian, or Mediterranean descent.
The G6PD enzyme is essential for producing NADPH, a co-enzyme critical for the secondary reduction pathway that methylene blue relies on. Therefore, administering methylene blue to a patient with G6PD deficiency will not only be ineffective but will also trigger a massive oxidative stress on their red blood cells, leading to a severe and potentially life-threatening hemolytic anemia.
For this reason, a G6PD assay should be performed before administering methylene blue if G6PD deficiency is suspected. Alternative treatments like ascorbic acid or exchange transfusion must be considered in these patients.
Frequently Asked Questions (FAQs)
How fast does methemoglobinemia occur?
The speed at which methemoglobinemia occurs can vary widely, but it often develops quite rapidly, especially in cases caused by drugs or toxins.
Onset Time
The onset of acquired methemoglobinemia can range from almost immediate to a few hours, depending on the substance and the dose. For example, a case study showed a patient developing severe methemoglobinemia within one minute after receiving a topical anesthetic. For other oxidizing agents, the onset is typically within 20-60 minutes of exposure.
Factors Influencing Speed
- The specific substance: Some oxidizing agents, like local anesthetics and nitrites, act very quickly.
- Dose and Route of Exposure: Higher doses and direct routes (like intravenous or nebulized administration) can lead to a much faster and more severe reaction.
- Individual Susceptibility: Patients with underlying conditions like anemia, cardiopulmonary disease, or genetic predispositions (like G6PD deficiency) can develop symptoms more quickly and at lower methemoglobin levels. This is especially true for infants, who are highly susceptible to triggers like nitrites in well water due to their immature enzyme systems.
What is the life expectancy of someone with methemoglobinemia?
The life expectancy of a person with methemoglobinemia depends entirely on the type of the condition, whether it’s congenital (inherited) or acquired and the severity of the specific case.
- Acquired Methemoglobinemia: For most patients with acquired methemoglobinemia, the prognosis is excellent with prompt and correct treatment. Since the condition is caused by an external agent, identifying and stopping the exposure, along with administering an antidote like methylene blue, typically leads to a full recovery without long-term health effects. The risk of death is primarily associated with very high methemoglobin levels (over 70%) or delays in diagnosis and treatment.
- Congenital Methemoglobinemia:
- Type I: This is a less severe form where the enzyme deficiency is limited to red blood cells. Patients often have chronic cyanosis but are otherwise healthy and have a normal life expectancy.
- Type II: This is a much more severe, generalized form of the disease. It affects multiple tissues and is associated with severe neurological problems and developmental delays. Tragically, children with Type II congenital methemoglobinemia often do not survive past early childhood.
- Hemoglobin M Disease: This is a chronic condition where patients have a structurally abnormal hemoglobin. They may have lifelong cyanosis but are generally asymptomatic and have a normal life expectancy.
How does vitamin C treat methemoglobinemia?
Vitamin C, also known as ascorbic acid, helps treat methemoglobinemia by acting as a non-enzymatic reducing agent. This means it directly donates electrons to the oxidized ferric (Fe3+) iron in methemoglobin, reducing it back to the functional ferrous (Fe2+) state. This process restores the hemoglobin’s ability to bind and transport oxygen.
While this mechanism is effective, it is much slower than the primary treatment, methylene blue, which works by accelerating a specific enzyme pathway. For this reason, ascorbic acid is typically reserved for patients with milder cases, or as a crucial alternative for individuals with a G6PD deficiency, who cannot safely be given methylene blue.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Ludlow JT, Wilkerson RG, Nappe TM. Methemoglobinemia. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537317/
- Rehman H. U. (2001). Methemoglobinemia. The Western journal of medicine, 175(3), 193–196. https://doi.org/10.1136/ewjm.175.3.193
- Iolascon, A., Bianchi, P., Andolfo, I., Russo, R., Barcellini, W., Fermo, E., Toldi, G., Ghirardello, S., Rees, D., Van Wijk, R., Kattamis, A., Gallagher, P. G., Roy, N., Taher, A., Mohty, R., Kulozik, A., De Franceschi, L., Gambale, A., De Montalembert, M., Forni, G. L., … SWG of red cell and iron of EHA and EuroBloodNet (2021). Recommendations for diagnosis and treatment of methemoglobinemia. American journal of hematology, 96(12), 1666–1678. https://doi.org/10.1002/ajh.26340
- Abhilash, Kundavaram Paul Prabhakar. Methemoglobinemia: When to Suspect and How to Treat. Current Medical Issues 17(4):p 125-128, Oct–Dec 2019. | DOI: 10.4103/cmi.cmi_55_19
- Lata, K., & Janardhanan, R. (2015). Methemoglobinemia: a diagnosis not to be missed. The American journal of medicine, 128(10), e45–e46. https://doi.org/10.1016/j.amjmed.2015.04.031