TL;DR
Malaria Life Cycle ▾
Malaria is caused by Plasmodium parasites transmitted through the bite of infected Anopheles mosquitoes. The parasite undergoes a complex life cycle involving both the mosquito and the human host:
- Mosquito Stage
- Human Stage
- Sexual Stage
Malaria Symptoms ▾
Malaria symptoms typically appear within a few weeks to a month after being bitten by an infected mosquito. They can range from mild to severe and include:
- Fever and chills
- Sweating
- Headache
- Muscle aches and fatigue
- Nausea and vomiting
Complications ▾
In severe cases, malaria can lead to life-threatening complications, such as:
- Severe Malarial Anemia
- Cerebral Malaria
Malaria Treatment ▾
Malaria treatment involves a two-pronged approach:
- Antimalarial Drugs
- Supportive Care
*Click ▾ for more information
Introduction
Severe malarial anemia is a life-threatening complication of malaria characterized by a dangerously low red blood cell count. Malaria poses a significant global health threat, infecting millions of individuals annually. The World Malaria Report 2015 estimated a staggering global burden in 2015, with cases ranging from 149 million to 303 million and a corresponding mortality rate estimated between 236,000 and 635,000. The WHO African Region disproportionately shoulders the disease burden, accounting for roughly 88% of all cases. The remaining cases are concentrated in the WHO South-East Asian Region (10%) and the WHO Eastern Mediterranean Region (2%).
Tiny single-celled organisms called Plasmodium parasites are the culprits behind malaria. These parasites hitch a ride on female Anopheles mosquitoes, which transmit them to humans through bites. Five Plasmodium species can infect humans: Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and the recently identified Plasmodium knowlesi.
While five Plasmodium species infect humans, two main ones cause the most significant disease:
- Plasmodium falciparum: The most prevalent and deadliest, responsible for the majority of severe malaria cases and deaths globally. It’s dominant in Africa but found elsewhere too.
- Plasmodium vivax: A major cause of relapsing malaria due to its ability to form dormant liver stages. While generally less severe than Plasmodium falciparum, it can still be serious. It’s more common outside sub-Saharan Africa.
The other three species are:
- Plasmodium malariae: Less common and typically causes milder illness.
- Plasmodium ovale: Similar to Plasmodium vivax in severity and geographical distribution.
- Plasmodium knowlesi: Recently identified and primarily found in Southeast Asia, it can cause severe malaria.
Malaria Life Cycle
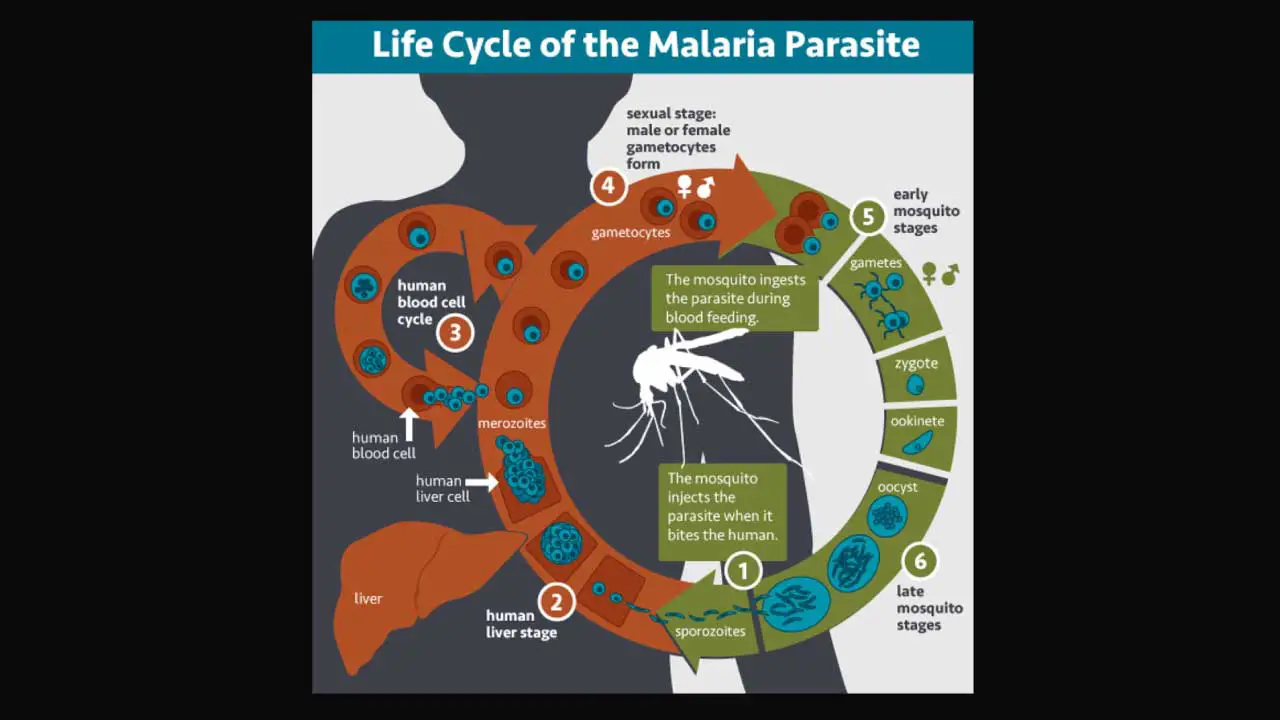
The malaria parasite undergoes a complex life cycle with distinct stages within both the mosquito (sexual phase) and the human host (asexual phase).
Mosquito Stage (Sporogonic Cycle) in Malaria Life Cycle
Transmission: During a blood meal, an infected female Anopheles mosquito injects sporozoites, the infectious stage of the parasite, into the human bloodstream.
Development Within the Mosquito (Sporogonic Cycle): Within minutes, sporozoites travel to the liver, where they burrow into liver cells. Inside the liver cells, they undergo a developmental process lasting 7-14 days, ultimately maturing into thousands of sporozoites again through a process called exo-erythrocytic schizogony. These new sporozoites migrate to the mosquito’s salivary glands, ready for transmission to another human when the mosquito bites again. In the case of Plasmodium vivax and Plasmodium ovale, some parasites can transform into dormant hypnozoites, which can remain dormant for months or years before initiating a new infection cycle, causing relapses.
Human Stage (Asexual Cycle) in Malaria Life Cycle
Replication Within Red Blood Cells (Erythrocytic Cycle): Within a few days, the liver cells rupture, releasing thousands of merozoites, the next asexual stage of the parasite. These merozoites invade red blood cells (RBCs), where they multiply rapidly. As they mature, they express proteins that allow them to adhere to the lining of blood vessels in organs like the brain, lungs, and placenta such as P. falciparum erythrocyte membrane protein 1.
Red Blood Cell Rupture and Cycle Continuation: After about 48 hours, the infected paratized RBCs burst, releasing even more merozoites, thereby perpetuating and promoting the blood-stage cycle. This cycle of invasion, replication, and rupture continues, causing the characteristic symptoms of malaria.
Pathogenesis and Transmission: The mere presence of the parasite might not be enough to cause severe illness. However, the repeated rupture of infected RBCs, release of parasite molecules, and the body’s immune response are believed to be the main culprits behind the severe complications seen in some cases.
Sexual Stages and Onward Transmission: Some merozoites develop into sexual forms called gametocytes. If a mosquito bites an infected person at this stage, it ingests these gametocytes. Inside the mosquito, the sexual cycle resumes, leading to the production of new sporozoites that can be transmitted to another human.
The Importance of Understanding the Malaria Life Cycle
Understanding the malaria life cycle is crucial for developing effective control strategies. By targeting both the mosquito and the parasite at different malaria life cycle stages, we can prevent transmission, reduce disease severity, and ultimately work towards malaria elimination.
Role of Platelets in Malaria Pathogenesis
Platelets are tiny blood cell fragments essential for normal blood clotting. They clump together to form plugs at wound sites, preventing excessive bleeding. However, in malaria, their role becomes more complex.
Physiological Role of Platelets
Normally, platelets circulate freely, patrolling for blood vessel damage. When a break occurs, they adhere to the injured area and release clotting factors to form a seal. This vital function prevents life-threatening blood loss from even minor injuries.
Disruption by Malaria Infection
Malaria disrupts this normal platelet function in several ways. Plasmodium parasites residing within infected red blood cells (PRBCs) can interact with platelets, leading to their activation. This activation can have both beneficial and detrimental effects.
Beneficial Effects: Parasite Killing
Activated platelets can bind to PRBCs. In some cases, this binding triggers the release of a protein called platelet factor 4 (PF4). PF4 has been shown to kill malaria parasites within the red blood cells, potentially offering a line of defense against the infection. Interestingly, further studies revealed that this killing required physical contact between the platelet and the infected red blood cell. Additionally, specific molecules on both the platelet (CD36) and the red blood cell (Duffy antigen) were found to be crucial for this platelet-mediated parasite killing, along with platelet factor 4 (PF4).
Detrimental Effects: Microvascular Complications
However, platelet activation can also have negative consequences. Malaria can activate cells lining the blood vessels (ECs) to release large strands of a protein called von Willebrand factor (VWF). These ultralarge VWF strands act like sticky threads that platelets can snag on. This tethering of platelets to the blood vessel wall can then lead to the clumping of infected red blood cells (PRBCs) in those areas. This clumping, called cytoadhesion and sequestration, ultimately blocks blood flow, particularly the small ones in vital organs like the brain. This phenomenon, known as microvascular occlusion, is a major contributor to severe complications in malaria.
The Complex Picture
The role of platelets in malaria is multifaceted. While they can potentially help fight the infection, their activation can also worsen it. It’s likely that this balance between beneficial and harmful effects might change throughout the course of the disease.
Supporting this complexity is the observation that people with severe malaria often have low platelet counts (thrombocytopenia) and is most marked in patients with severe Plasmodium falciparum infection. The extent of thrombocytopenia correlates with parasite density, severity of malaria infection, and clinical outcomes. This could be due to excessive platelet activation and destruction, or possibly the parasites using platelets for their own survival.
Malaria Symptoms
Malaria symptoms can vary depending on the severity of the infection and the specific Plasmodium species involved.
Early Flu-like Symptoms (Common)
- Fever and chills
- Sweating
- Headache
- Muscle aches and fatigue
- Nausea and vomiting
- General feeling of discomfort
These flu-like symptoms are often the first signs and can appear within a few weeks to a month after being bitten by an infected mosquito.
Symptoms of Severe Malaria (More concerning, require immediate medical attention)
- Severe anemia (pallor, fatigue, shortness of breath)
- Jaundice (yellowing of the skin and whites of the eyes)
- Seizures or coma (cerebral malaria)
- Kidney failure
- Difficulty breathing
- Dark or bloody urine
The symptoms of severe malaria can vary depending on the age of the patient. In young children (under 2 years old), severe anemia is the most common complication. Older children are more likely to experience seizures and cerebral malaria (coma). Adults with severe malaria can develop a wider range of complications, including acute kidney failure, fluid buildup in the lungs (acute pulmonary edema), liver problems, and cerebral malaria.
Complications of Malaria
Severe Malarial Anemia
Severe malarial anemia (SMA) is a life-threatening complication of malaria, characterized by a dangerously low red blood cell count, hemoglobin concentration below 5 g/dL or a hematocrit (percentage of red blood cells in whole blood) below 15% in children under 12 years old (below 7 g/dL and 20% respectively in adults). It’s considered the most common and serious complication of malaria, potentially claiming more lives globally than cerebral malaria. Unlike the dramatic symptoms of cerebral malaria, SMA can be silent and chronic, making it less well-understood.
Mechanisms of Red Blood Cell Destruction
Two main processes contribute to the severe anemia in malaria:
- Increased Destruction of Non-Parasitized Red Blood Cells: Malaria infection alters the surface and structure of healthy red blood cells, making them susceptible to destruction by the body’s own defense mechanisms. This destruction can be triggered by various factors, including oxidation, reduced flexibility of the red blood cells, and the presence of specific molecules on their surface. The immune system may also contribute through complement activation, autoantibodies, and immune complexes. Studies have shown that the body’s reticuloendothelial system (RES) becomes more active during malaria infection. This increased activity is linked to the destruction of healthy red blood cells (not just those infected with parasites) and may also contribute to the decrease in platelets observed in malaria patients (thrombocytopenia).
- Decreased Red Blood Cell Production: In severe malaria caused by P. falciparum, doctors often observe low levels of reticulocytes (immature red blood cells) in the bloodstream. This suggests another mechanism besides red blood cell destruction might be contributing to severe malarial anemia – a suppression of red blood cell production in the bone marrow (erythropoietic suppression).While the exact mechanism is still being investigated, it’s believed to be linked to the release of inflammatory molecules like TNF and IFN-γ during the early stages of infection. These molecules may make red blood cell precursors less responsive to erythropoietin, a hormone that stimulates red blood cell production.
Interestingly, even after the malaria parasites are cleared from the bloodstream, anemia can persist. This suggests that the increased red blood cell destruction might continue beyond the active infection phase.
Cerebral Malaria
Cerebral malaria (CM) is the most severe neurological complication of Plasmodium falciparum infection. It’s characterized by a decline in mental alertness, progressing to coma and seizures.
Pathophysiology of Brain Involvement
The leading theory for CM pathogenesis involves the accumulation of infected red blood cells (PRBCs) within the small blood vessels of the brain (microvessels). This phenomenon, known as sequestration, disrupts blood flow and oxygen delivery to brain tissue.
- Sequestration: Mature PRBCs infected with P. falciparum express sticky molecules on their surface that allow them to cling to the lining of brain microvessels. This adhesion process leads to clumps of infected and uninfected red blood cells, blocking blood flow and causing oxygen deprivation (hypoxia) in surrounding brain tissue.
- Blood-Brain Barrier Disruption: The accumulation of PRBCs and the activated immune response can damage the blood-brain barrier, a specialized layer of cells that normally protects the brain from harmful substances in the bloodstream. This further contributes to brain inflammation and swelling.
Laboratory Investigations for Malaria
Diagnosing malaria relies on laboratory tests to detect the presence of the Plasmodium parasites in a patient’s blood.
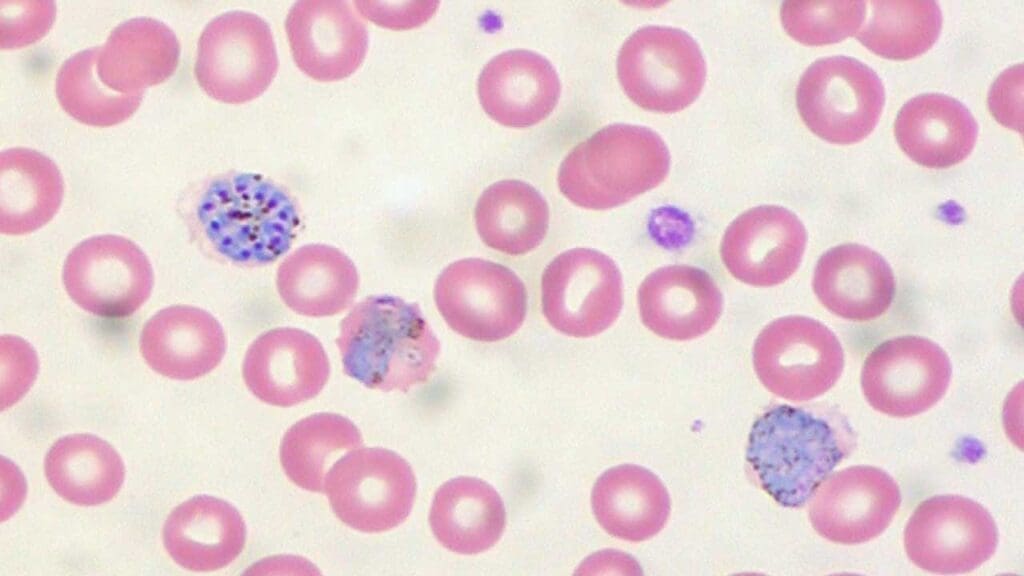
- Microscopic Blood Smear: This is the “gold standard” for malaria diagnosis. A thin and thick blood film is stained and examined under a microscope by a trained professional. This allows for visualization of the parasite itself within red blood cells, identification of the specific Plasmodium species, and assessment of parasite density (number of parasites per volume of blood).
- Rapid Diagnostic Test (RDT): This is a rapid point-of-care test that uses immunochromatography technology. It detects the presence of specific proteins (antigens) produced by the Plasmodium parasites. RDTs are quick, convenient, and can be used in resource-limited settings. However, they may not be as sensitive as blood smears, particularly in low parasite density infections, and cannot differentiate between different Plasmodium species.
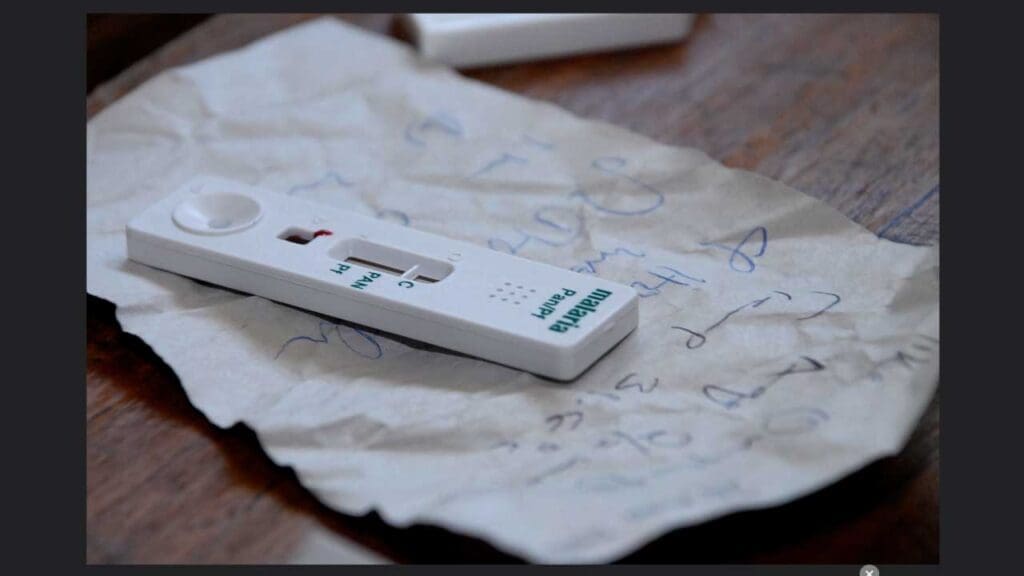
Additional Tests
In some cases, additional laboratory tests may be ordered to assess the severity of the infection and potential complications, such as:
- Full Blood Count (FBC): This measures the levels of red blood cells, white blood cells, and platelets, which can be helpful in identifying anemia, abnormal white blood cell counts, and potential thrombocytopenia (low platelet count) associated with malaria.
- Electrolytes and Kidney Function Tests: These can assess electrolyte imbalances and potential kidney dysfunction that can occur in severe malaria.
- Liver Function Tests: These may be done to check for liver damage, which can be a complication of malaria.
Malaria Treatment
Malaria treatment involves a two-pronged approach: targeting the malaria parasites with medications (antimalarials) and providing supportive care to manage the patient’s overall health.
Antimalarial Drugs
These medications are the cornerstone of malaria treatment, aiming to eliminate the Plasmodium parasites from the bloodstream.
- Artemisinin Derivatives: These are the most effective and widely used antimalarial drugs for malaria treatment. They work by rapidly killing the malaria parasites within the red blood cells. Examples include artesunate and artemether.
- Other Antimalarial Classes: Other classes of drugs for malaria treatment target different stages of the parasite’s life cycle. Some examples include:
- Aminoquinolines: These drugs (e.g., chloroquine) were once a mainstay of treatment but have become less effective due to widespread parasite resistance.
- Antifolics: These drugs (e.g., sulfadoxine-pyrimethamine) block the parasite’s ability to synthesize essential folic acid, hindering its growth and replication.
- Others: Several other antimalarial drug classes exist, but their use is more limited due to factors like potential side effects or specific targeting of parasite stages.
- Combination Therapy: The critical importance of combination therapy cannot be overstated in malaria treatment. Using two or more antimalarial drugs with different mechanisms of action significantly reduces the risk of the parasite developing resistance to any single medication. This approach ensures a more effective and faster cure.
- Drug Resistance: The emergence of drug-resistant malaria parasites is a growing concern in malaria treatment. Artemisinin resistance, while still relatively rare, poses a significant threat. Continuous monitoring of resistance patterns and development of new antimalarial drugs are crucial for maintaining effective treatment options.
Supportive Management
While medications target the parasites, supportive care is essential for managing the patient’s overall health and addressing potential complications in malaria treatment.
- Fluid Resuscitation: Malaria can lead to dehydration and electrolyte imbalances. Rehydration with intravenous fluids is often necessary to restore fluid balance and improve blood flow to vital organs.
- Electrolyte Balance: Electrolyte imbalances (e.g., low potassium or sodium) can occur due to vomiting, diarrhea, or excessive sweating. Correcting these imbalances is crucial for proper muscle and nerve function.
- Blood Transfusions (if needed): In severe cases of malaria with significant anemia, blood transfusions may be required to replace lost red blood cells and improve oxygen delivery to tissues.
- Supportive Care for Specific Complications: Depending on the severity of the infection and the presence of complications like cerebral malaria, additional supportive measures like respiratory support or anticonvulsant medications might be necessary.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Jamie M. O’Sullivan, James S. O’Donnell; Platelets in malaria pathogenesis. Blood 2018; 132 (12): 1222–1224. doi: https://doi.org/10.1182/blood-2018-08-865618.
- Schofield, L., Grau, G. Immunological processes in malaria pathogenesis. Nat Rev Immunol 5, 722–735 (2005). https://doi.org/10.1038/nri1686
- https://www.who.int/news-room/fact-sheets/detail/malaria
- Arbeitskreis Blut, Untergruppe «Bewertung Blutassoziierter Krankheitserreger». Malaria. Transfus Med Hemother. 2009;36(1):48-60. doi: 10.1159/000197327. PMID: 21048821; PMCID: PMC2928834.
- Wassmer SC, Taylor TE, Rathod PK, Mishra SK, Mohanty S, Arevalo-Herrera M, Duraisingh MT, Smith JD. Investigating the Pathogenesis of Severe Malaria: A Multidisciplinary and Cross-Geographical Approach. Am J Trop Med Hyg. 2015 Sep;93(3 Suppl):42-56. doi: 10.4269/ajtmh.14-0841. Epub 2015 Aug 10. PMID: 26259939; PMCID: PMC4574273.
- White NJ. Anaemia and malaria. Malar J. 2018 Oct 19;17(1):371. doi: 10.1186/s12936-018-2509-9. PMID: 30340592; PMCID: PMC6194647.
- Fitri LE, Widaningrum T, Endharti AT, Prabowo MH, Winaris N, Nugraha RYB. Malaria diagnostic update: From conventional to advanced method. J Clin Lab Anal. 2022 Apr;36(4):e24314. doi: 10.1002/jcla.24314. Epub 2022 Mar 4. PMID: 35247002; PMCID: PMC8993657.
- Ippolito MM, Moser KA, Kabuya JB, Cunningham C, Juliano JJ. Antimalarial Drug Resistance and Implications for the WHO Global Technical Strategy. Curr Epidemiol Rep. 2021;8(2):46-62. doi: 10.1007/s40471-021-00266-5. Epub 2021 Mar 14. PMID: 33747712; PMCID: PMC7955901.
- Draper SJ, Sack BK, King CR, Nielsen CM, Rayner JC, Higgins MK, Long CA, Seder RA. Malaria Vaccines: Recent Advances and New Horizons. Cell Host Microbe. 2018 Jul 11;24(1):43-56. doi: 10.1016/j.chom.2018.06.008. PMID: 30001524; PMCID: PMC6054918.