TL;DR
What is tissue plasminogen activator (tPA)? ▾
- Tissue plasminogen activator (tPA) is a drug used to dissolve blood clots.
How does it work? ▾
- Works by activating plasminogen, which breaks down fibrin in clots.
Medical uses ▾
- Primarily used for ischemic stroke, heart attack, and pulmonary embolism.
- Can also be used for certain types of blood clots in other locations.
Effectiveness ▾
- Improves outcomes for patients with blood clot-related conditions.
- Reduces tissue damage and mortality rates.
Risks ▾
- Primary risk is bleeding.
- Other potential side effects include allergic reactions and reperfusion injury.
Administration
- Given intravenously.
- Requires careful monitoring due to bleeding risks.
*Click ▾ for more information
Introduction
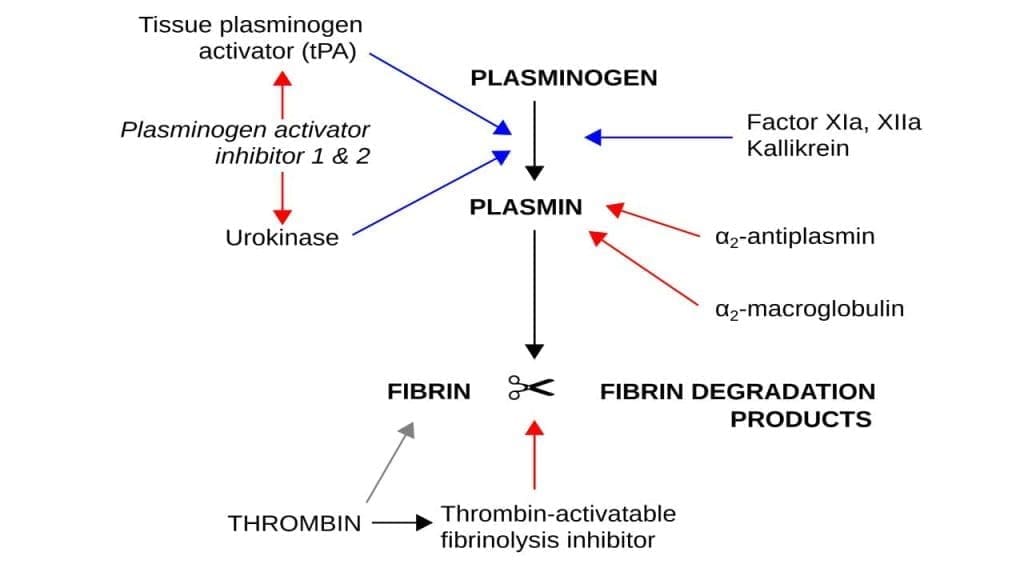
Tissue plasminogen is a crucial component of the fibrinolytic system, which is responsible for breaking down blood clots. Tissue plasminogen is a precursor protein produced by the liver and found circulating in the bloodstream. It acts as a precursor to the active enzyme, plasmin. Tissue plasminogen is essentially an inactive form of the enzyme that dissolves blood clots.
Tissue Plasminogen Activator (tPA) Function
When a blood clot forms, tissue plasminogen becomes embedded within its structure. This positioning is vital for the subsequent activation process. Tissue plasminogen activator (tPA) converts tissue plasminogen into its active form, plasmin. This conversion primarily occurs on the surface of the blood clot, ensuring targeted clot breakdown. Plasmin, the activated form of tissue plasminogen, is the primary enzyme responsible for degrading the fibrin network within the clot. This breakdown leads to the dissolution of the clot and restoration of blood flow.
Blood clots (thrombus), essential for wound healing, can become dangerous when they form in the wrong place or don’t dissolve properly. These unwanted clots can block blood flow to vital organs, causing serious complications. For instance, a clot in the brain can lead to stroke, while one in the heart can cause a heart attack. In the lungs, a blood clot can result in a pulmonary embolism, a potentially fatal condition.
When a blood clot obstructs blood flow to vital organs, it can lead to severe complications. Some of the most critical conditions caused by blood clots include:
- Heart attack: A clot blocking blood flow to the heart muscle, causing tissue damage and potentially leading to cardiac arrest.
- Stroke: A clot obstructing blood flow to the brain, resulting in brain cell death and neurological impairment.
- Pulmonary embolism: A clot that travels to the lungs, blocking blood flow and impairing oxygen exchange.
- Deep vein thrombosis (DVT): A clot forming in a deep vein, often in the legs, which can dislodge and travel to the lungs (pulmonary embolism).
These conditions are medical emergencies requiring immediate intervention.
A Brief History of Tissue Plasminogen Activator Development
The development of tissue plasminogen activator (tPA) as a therapeutic agent is a significant milestone in medical history.
- Early attempts: Prior to tissue plasminogen activator (tPA), substances like streptokinase and urokinase were used to dissolve blood clots. However, these agents carried significant risks of bleeding.
- Discovery of tissue plasminogen activator (tPA): In the 1970s, researchers discovered that certain cancer cells produced large amounts of tissue plasminogen activator (tPA). This enzyme showed promise in dissolving clots without the same level of bleeding risk as earlier treatments.
- Development as a drug: Extensive research and development followed to create a purified and stable form of tissue plasminogen activator (tPA) suitable for clinical use.
- Clinical trials: Rigorous clinical trials were conducted to assess the safety and efficacy of tissue plasminogen activator (tPA) in treating conditions like heart attack and stroke.
- FDA approval: Based on successful trials, tissue plasminogen activator (tPA) was approved for medical use in the early 1990s, revolutionizing the treatment of acute ischemic stroke and heart attack.
The development of tissue plasminogen activator (tPA) marked a significant advancement in the management of blood clot-related conditions, offering hope to countless patients.
Medical Applications of Tissue Plasminogen Activator
While there’s only one primary type of tPA – tissue plasminogen activator – there are different formulations and modifications of this enzyme used in clinical practice.
Each has unique characteristics and applications.
Primary Types of Tissue Plasminogen Activator (tPA)
- Alteplase: This is the most commonly used form of tissue plasminogen activator (tPA). It’s a genetically engineered version of the naturally occurring human tissue plasminogen activator (tPA). Alteplase is used to treat ischemic stroke, myocardial infarction (heart attack), pulmonary embolism, and certain cases of blood clots in central venous catheters.
- Reteplase: A modified form of alteplase, reteplase has a longer half-life, allowing for less frequent dosing. It’s primarily used in the treatment of acute myocardial infarction.
- Tenecteplase: Another modified form of alteplase, tenecteplase has an even longer half-life than reteplase. It’s also used in the treatment of acute myocardial infarction.
Primary Conditions Treated with Tissue Plasminogen Activator (tPA)
Ischemic Stroke
Ischemic stroke occurs when a blood clot blocks blood flow to the brain. This deprivation of oxygen and nutrients can cause significant brain damage. Tissue plasminogen activator (tPA) is administered intravenously to dissolve the clot and restore blood flow to the affected area. It’s crucial to administer tissue plasminogen activator (tPA) within a narrow time window (usually within 4.5 hours) of symptom onset to maximize its effectiveness.
Pulmonary Embolism
A pulmonary embolism is a blockage in one or more of the pulmonary arteries, often caused by a blood clot that travels from the legs (deep vein thrombosis) to the lungs. Tissue plasminogen activator (tPA) can be used to dissolve the clot and improve blood flow to the lungs. However, its use in pulmonary embolism is more complex and often reserved for severe cases or when other treatments fail.
Myocardial Infarction (Heart Attack)
A heart attack occurs when blood flow to the heart muscle is blocked, often by a blood clot. Tissue plasminogen activator (tPA) can be used to dissolve the clot and restore blood flow to the heart. Similar to stroke, time is of the essence, and tissue plasminogen activator (tPA) should be administered as soon as possible. However, primary percutaneous coronary intervention (PCI) is generally preferred when available, as it offers better outcomes.
It’s important to note that while tissue plasminogen activator (tPA) is a valuable tool in treating these conditions, it carries risks, primarily bleeding. Therefore, its use is carefully considered and monitored.
Benefits and Risks of Tissue Plasminogen Activator (tPA)
Effectiveness of Tissue Plasminogen Activator (tPA) in Improving Outcomes
Tissue plasminogen activator (tPA) has significantly improved outcomes for patients suffering from acute ischemic stroke, myocardial infarction, and pulmonary embolism. When administered within appropriate time windows, tissue plasminogen activator (tPA) can:
- Reduce brain damage in stroke patients by restoring blood flow.
- Limit heart muscle damage in heart attack patients by reopening blocked coronary arteries.
- Improve breathing and oxygenation in pulmonary embolism patients by dissolving clots obstructing blood flow to the lungs.
These benefits translate into reduced mortality rates, decreased disability, and improved quality of life for affected patients.
Potential Side Effects and Complications
While tissue plasminogen activator (tPA) is a life-saving drug, it carries inherent risks, primarily related to bleeding.
- Hemorrhage: This is the most common and serious complication of tissue plasminogen activator (tPA). It can occur at the site of injury, in the brain, or in other organs.
- Allergic reactions: Although rare, allergic reactions to tissue plasminogen activator (tPA) can occur.
Other potential complications include:
- Reperfusion injury: Damage to tissues that occurs when blood flow is restored after a period of ischemia.
- Increased intracranial pressure: This can occur in stroke patients.
Risk-Benefit Assessment and Careful Monitoring
The decision to administer tissue plasminogen activator (tPA) involves a careful evaluation of the potential benefits against the risks. Factors such as the severity of the condition, the time elapsed since symptom onset, and the patient’s overall health status are considered.
Continuous monitoring of patients receiving tissue plasminogen activator (tPA) is essential to detect and manage potential complications early. This includes close observation for signs of bleeding, blood pressure monitoring, and neurological assessments (in stroke patients).
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Saba HI, Roberts HR. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management (Wiley Blackwell). 2014.
- DeLoughery TG. Hemostasis and Thrombosis 4th Edition (Springer). 2019.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.
- Kaushansky K, Levi M. Williams Hematology Hemostasis and Thrombosis (McGraw-Hill). 2017.
- Wang DZ, Rose JA, Honings DS, Garwacki DJ, Milbrandt JC. Treating acute stroke patients with intravenous tPA. The OSF stroke network experience. Stroke. 2000 Jan;31(1):77-81. doi: 10.1161/01.str.31.1.77. PMID: 10625719.
- Califf RM, Mantell S, Westawski C, Bride W, Honan MB, Bellinger RL, Wall TC. Experience with the use of tPA in the treatment of acute myocardial infarction. Ann Emerg Med. 1988 Nov;17(11):1176-89. doi: 10.1016/s0196-0644(88)80064-7. PMID: 2973270.