TL;DR
Hemophilia, the most common severe, inherited bleeding disorder, represents a failure in the secondary hemostatic mechanism. It is an X-linked recessive disorder characterized by a deficiency or dysfunction of a specific plasma coagulation factor, leading to prolonged and spontaneous bleeding, most notably into joints and muscles.
- Clinical features ▾:
- Joint and soft tissue bleeds
- Excessive bruising
- Recurrent painful hemarthrosis and muscle hematomas may lead to progressive joint deformity and disability if poorly treated
- Laboratory diagnosis ▾:
- Treatment and Management ▾:
- Factor VIII replacement therapy depending on severity
- DDAVP in mild – moderate cases
- Self-treatment prophylactic factor VIII
- Recombinant factor VIIa or FEIBA if high inhibitory antibodies
*Click ▾ for more information
What is hemophilia?
Hemophilia is a group of inherited bleeding disorders characterized by a deficiency or dysfunction of specific clotting factors, leading to impaired hemostasis. It is primarily a failure of secondary hemostasis, meaning the initial platelet plug formation (primary hemostasis) occurs normally, but the subsequent stabilization of that plug with a fibrin mesh is defective. This results in prolonged and recurrent bleeding, often spontaneously into muscles and joints.
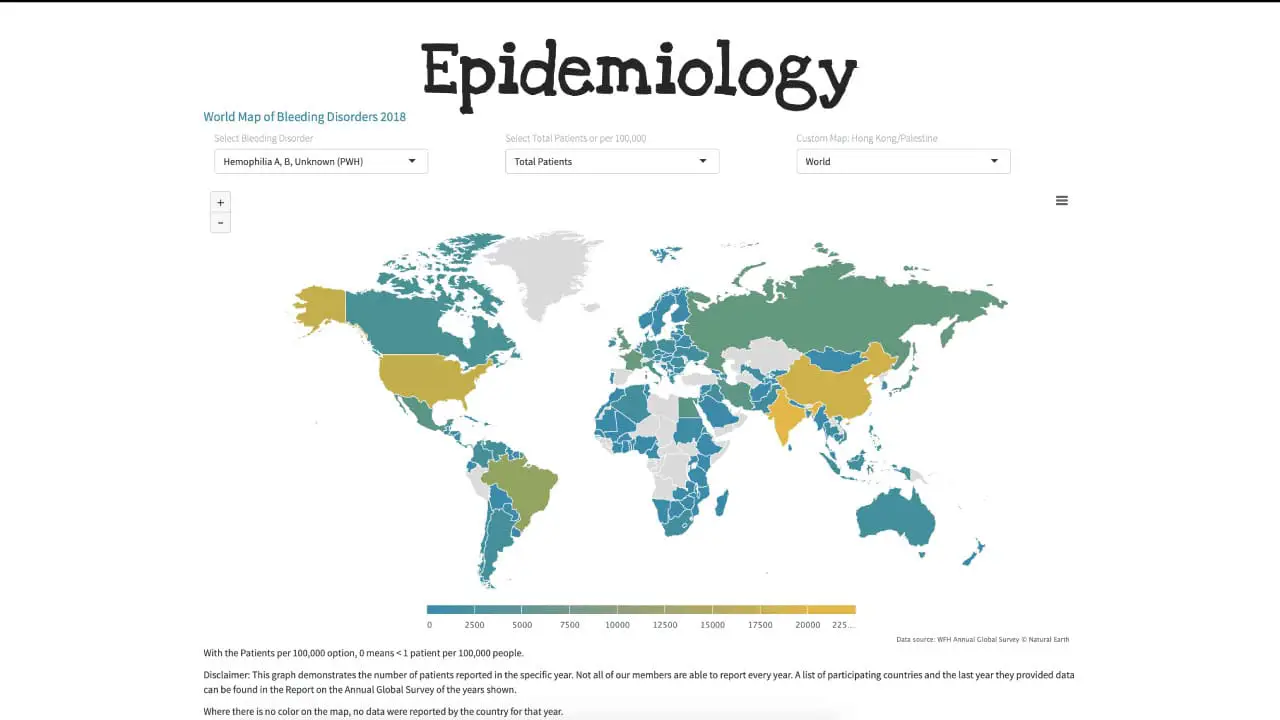
Hemophilia A is the most common of the hereditary clotting factor deficiencies. The prevalence is approximately 30 – 100 per million population and can be found almost all over in the world. The incidence of hemophilia B on the other hand is one-fifth of that of hemophilia A.
Classification of Hemophilia
Hemophilia is classified into two major, clinically indistinguishable types based on the missing factor.
| Classification | Deficient Factor | Genetics | Role in Coagulation |
|---|---|---|---|
| Hemophilia A (Classic Hemophilia) | Factor VIII (FVIII) | Caused by mutations in the F8 gene on the X chromosome. The most common severe mutation is a large inversion involving intron 22, resulting in zero production of functional FVIII | FVIII is a critical cofactor that, when activated (FVIIIa), binds to activated Factor IX (FIXa) to form the tenase complex (FIXa-FVIIIa). This complex efficiently activates Factor X (FX) in the intrinsic pathway, dramatically accelerating the coagulation cascade. Its absence severely cripples thrombin generation. |
| Hemophilia B (Christmas Disease) | Factor IX (FIX) | Caused by mutations in the F9 gene on the X chromosome | IX is a serine protease that, when activated (FIXa), forms the catalytic component of the tenase complex. Its deficiency prevents the efficient activation of FX |
In addition to type, hemophilia is universally classified by severity, which directly correlates with the amount of functional clotting factor activity present in the patient’s plasma. Both deficiencies result in defective thrombin generation, leading to an inability to form a stable fibrin clot at the site of vascular injury.
| Severity Level | Factor Activity Level |
| Severe | < 1% of normal activity |
| Moderate | 1%–5% of normal activity |
| Mild | > 5%–40% of normal activity |
Causes and Inheritance of Hemophilia
Genetic Basis: X-Linked Recessive Inheritance
Hemophilia A and Hemophilia B are inherited in an X-linked recessive pattern. This means the genes responsible for producing the functional clotting factors (FVIII and FIX) are located on the X chromosome.
- Carrier Status: Females with one affected X chromosome and one healthy X chromosome are termed carriers. While historically considered asymptomatic, many carriers may have factor levels between 40% and 60% and can experience mild bleeding symptoms, necessitating the term “symptomatic carriers” or “obligate carriers.”
- Mechanism in Males (XY): Males have only one X chromosome. If that X chromosome carries the mutated gene, they will express the disorder because they lack a second, healthy X chromosome to compensate. Therefore, a male with the affected gene is a male with hemophilia.
- Mechanism in Females (XX): Females have two X chromosomes. Because the disease is recessive, a female must inherit the affected gene on both X chromosomes to have severe hemophilia (a rare event, often associated with specific chromosomal abnormalities). Typically, if a female inherits one mutated gene, the functional gene on the other X chromosome provides enough factor activity to prevent severe bleeding.

Gene Location
The specific location of the mutation dictates the type of hemophilia.
- Hemophilia A (FVIII Deficiency): Caused by mutations in the Factor 8 gene (F8), located near the tip of the long arm of the X chromosome (Xq28). The most common severe mutation in Hemophilia A is a large inversion that disrupts the gene structure, known as the intron 22 inversion.
- Hemophilia B (FIX Deficiency): Caused by mutations in the Factor 9 gene (F9), also located on the long arm of the X chromosome (Xq27). Mutations here are typically point mutations or small deletions.
Acquired Hemophilia
While the vast majority of cases are inherited, students should be aware of acquired hemophilia (AH), a rare, but severe, bleeding disorder. Acquired hemophilia requires entirely different and often more urgent management than inherited hemophilia because the bleeding is usually severe and life-threatening, and replacement factor therapy may be ineffective due to the presence of inhibitors.
AH is an autoimmune disorder where the patient’s immune system spontaneously produces autoantibodies (inhibitors) against their own circulating FVIII. It typically affects older individuals (median age > 60 years) and often occurs in association with underlying conditions like malignancy, autoimmune diseases (e.g., rheumatoid arthritis), or post-partum (pregnancy-related). Critically, the patient has no prior personal or family history of a bleeding disorder.
Pathophysiology of Hemophilia
The central issue in hemophilia is the inefficient generation of thrombin, the enzyme essential for converting fibrinogen into the stable fibrin clot.
Hemostasis
Hemostasis, the process of stopping bleeding, has two phases:
- Primary Hemostasis: Involves the vasoconstriction and the formation of the initial platelet plug. Since Factors VIII and IX are not involved in this phase, it is typically normal in hemophilia.
- Secondary Hemostasis (Coagulation Cascade): Involves the intricate, enzyme-driven cascade that stabilizes the platelet plug by generating a robust, cross-linked fibrin mesh. This phase is defective in hemophilia.
The fundamental defect is the failure to form enough stable fibrin clots due to a lack of either Factor VIII (Hemophilia A) or Factor IX (Hemophilia B).
The Intrinsic Pathway and the Tenase Complex
Hemophilia disrupts the intrinsic pathway of the coagulation cascade. The key reaction affected is the formation of the Factor X-activating complex, commonly known as the intrinsic tenase complex. Factor IXa (activated Factor IX) combines with Factor VIIIa (activated Factor VIII) on the surface of activated platelets. This complex acts as a powerful catalyst. The intrinsic tenase complex (Factor IXa + Factor VIIIa) rapidly activates Factor X to Factor Xa. Factor Xa is the starting point of the common pathway.
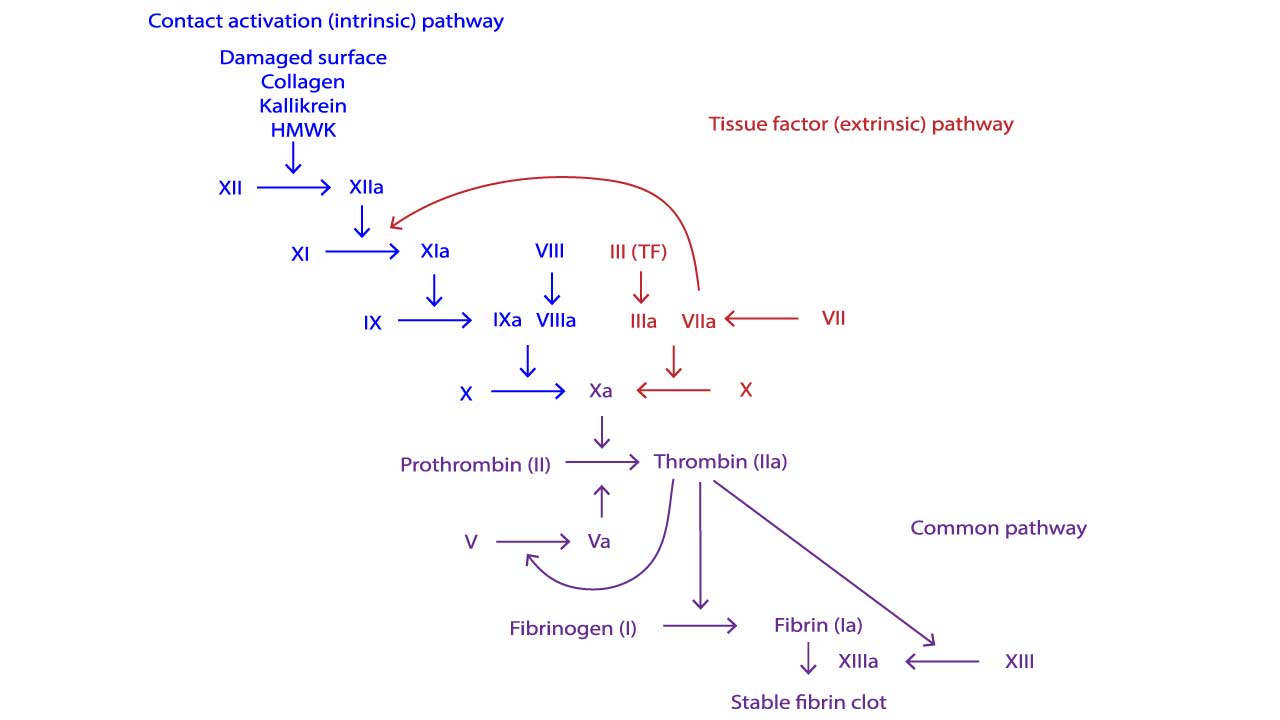
The Hemophilia Bottleneck
- Hemophilia A (FVIII Deficiency): Factor VIII (FVIII) serves as a cofactor for Factor IXa. It does not possess enzymatic activity itself but dramatically accelerates the rate at which Factor IXa can activate Factor X. Without sufficient FVIII, Factor IXa’s activity is essentially non-existent, creating a severe bottleneck in the cascade.
- Hemophilia B (FIX Deficiency): Factor IX is the enzyme in the complex. Without sufficient FIX, the crucial enzymatic step that cleaves and activates Factor X cannot occur.
In both A and B, the downstream generation of Factor Xa is critically slowed, leading to insufficient thrombin generation.
Consequence of the Defects
The defect in secondary hemostasis explains the characteristic bleeding pattern of hemophilia.
- Prolonged Bleeding: Since the fibrin mesh forms slowly, bleeding continues long after the initial injury (in contrast to platelet disorders, where bleeding often starts immediately and resolves relatively quickly).
- Deep Tissue Bleeding (The Clinical Hallmark): The primary platelet plug is structurally weak and easily dislodged by normal blood pressure or movement, especially in large, unsupported vessels. This leads to bleeding into deep, high-pressure spaces:
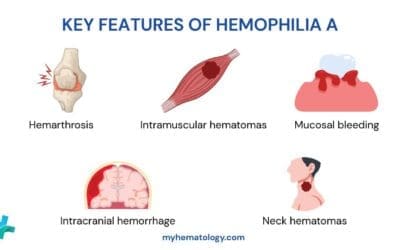
- Hemarthrosis: Bleeding into joint spaces (ankles, knees, elbows). This is the most common and damaging manifestation.
- Hematomas: Deep muscle bleeding.
- Intracranial Hemorrhage (ICH): The most life-threatening complication, resulting from the failure to seal vessels in the brain or skull effectively.
Signs and Symptoms of Hemophilia
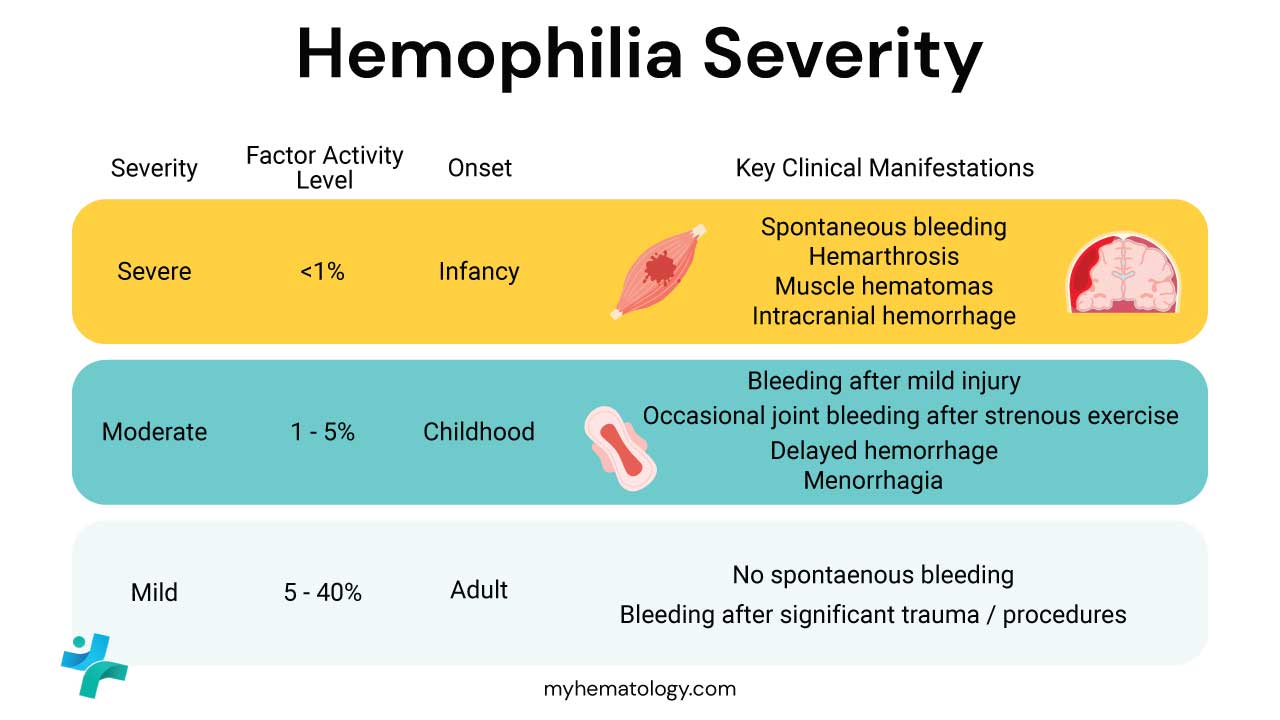
The clinical presentation of hemophilia is highly variable, dictated almost entirely by the residual level of functional clotting factor (Factor VIII or IX) activity in the patient’s plasma.
| Severity Level | Factor Activity Level | Typical Bleeding Pattern |
|---|---|---|
| Severe | < 1% | Spontaneous bleeding without discernible cause, beginning early in life (often before 2 years old). Requires prophylactic treatment. |
| Moderate | 1%–5% | Infrequent spontaneous bleeding; significant bleeding typically follows minor trauma, dental procedures, or surgical interventions. |
| Mild | > 5%–40% | Rarely or never spontaneous. Bleeding occurs only after major trauma, surgery, or invasive procedures. May not be diagnosed until adulthood. |
Sites of Bleeding (Crucial for Diagnosis)
The hallmark of hemophilia is deep bleeding into enclosed spaces, particularly the joints and muscles, as opposed to the superficial mucosal bleeding characteristic of platelet disorders (like Von Willebrand disease).
Musculoskeletal Bleeding (The Primary Concern)
- Hemarthrosis (Joint Bleeding): This is the signature symptom of severe hemophilia and the leading cause of morbidity. Bleeding most commonly affects the target joints (those that bleed repeatedly), especially the knees, elbows, and ankles.
- Progression: Bleeding into the joint capsule causes pain, swelling, warmth, and limited range of motion.
- Long-Term Impact: Recurrent hemarthrosis leads to chronic inflammation, cartilage destruction, and bone erosion, culminating in hemophilic arthropathy—a debilitating, destructive joint disease.
- Deep Muscle Hematomas: Bleeding into large muscle groups (e.g., iliopsoas, thigh). These can cause compartmental syndrome (rarely) or, more commonly, nerve compression, leading to significant pain and potential paralysis (e.g., femoral nerve compression from an iliopsoas bleed).
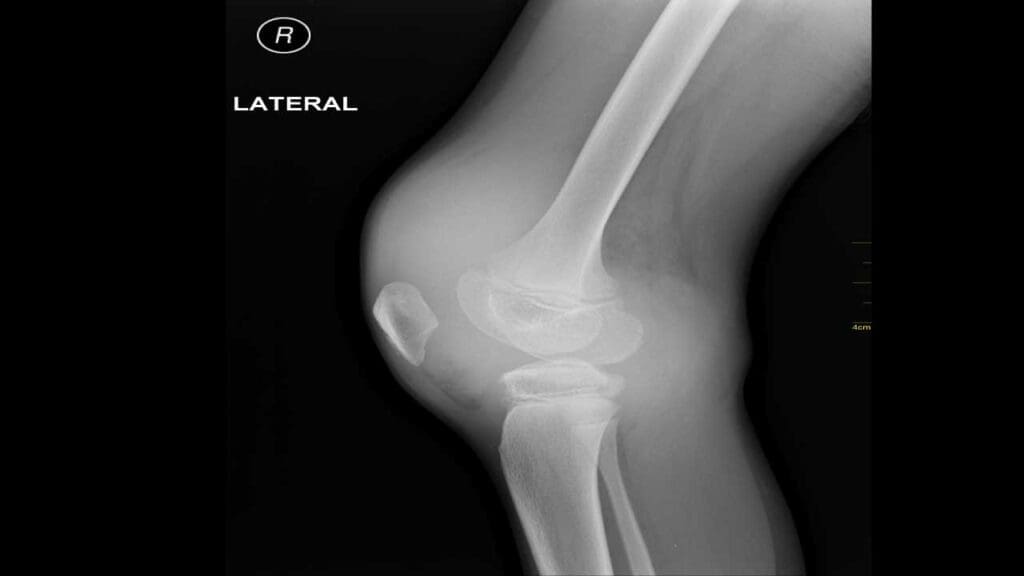
Mucosal and Superficial Bleeding
While less diagnostic than joint bleeding, patients often present with:
- Epistaxis (Nosebleeds): Usually manageable but can be persistent.
- Gingival Bleeding: Prolonged bleeding following dental extraction or procedures.
- Easy Bruising (Ecchymoses): Large, tender bruises due to subcutaneous tissue bleeding after minor bumps.
Life-Threatening Hemorrhage (Emergencies)
Any major bleeding event requires immediate factor replacement.
- Intracranial Hemorrhage (ICH): The leading cause of death and disability in patients with hemophilia. Bleeding can be spontaneous or follow head trauma, presenting with severe headache, vomiting, altered consciousness, or seizures. ICH must be assumed until ruled out.
- Retroperitoneal Bleeding: Bleeding behind the peritoneum, often involving the psoas muscle, which can lead to significant blood loss and nerve compression.
- Gastrointestinal Bleeding: Bleeding anywhere along the GI tract.
- Neck/Throat Hematoma: Bleeding in the soft tissues of the neck can rapidly compromise the airway, constituting an acute emergency.
Infancy Signs
In severe hemophilia, the first signs of the disorder typically appear within the first two years of life.
- Prolonged Bleeding after Circumcision: This is frequently the first presentation that prompts diagnosis.
- Cephalohematoma: Bleeding under the scalp following delivery.
- Excessive Bruising: Noticed as the infant becomes mobile and starts to crawl or walk, leading to recurrent ecchymoses.
- Muscle Bleeding: Occasionally seen as the infant begins to cruise or walk.
Laboratory Investigations and Diagnosis of Hemophilia
The diagnosis of hemophilia relies on combining a high index of clinical suspicion (e.g., recurrent hemarthroses, family history) with a characteristic pattern of coagulation test results.
Screening Coagulation Tests
Initial diagnosis is made using standard coagulation tests that evaluate the different pathways of the hemostatic system.
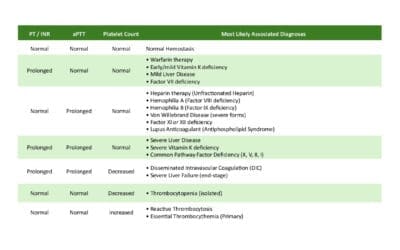
- Activated Partial Thromboplastin Time (aPTT): Prolonged. Since Factor VIII (Hemophilia A) and Factor IX (Hemophilia B) are integral components of the Intrinsic Pathway, their deficiency dramatically slows down the time required for plasma to clot. The degree of prolongation generally correlates with the severity of the factor deficiency.
- Prothrombin Time (PT): Normal.
- Thrombin Time (TT): Normal. The deficiency does not directly affect the structure or function of fibrinogen or thrombin itself.
- Platelet Count and Bleeding Time: Normal
Confirmatory Diagnostic Tests
If the aPTT is prolonged and the PT is normal, a quantitative factor assay is mandatory to confirm the diagnosis, determine the type of hemophilia, and assess its severity.
Specific Factor Assays
These assays directly measure the functional activity of a specific clotting factor in the patient’s plasma.
- A low Factor VIII activity confirms Hemophilia A.
- A low Factor IX activity confirms Hemophilia B.
- Severity Determination: The result of the factor assay, expressed as a percentage of normal activity, directly determines the clinical severity:
- Severe: < 1%
- Moderate: 1%–5%
- Mild: > 5%–40%
Genetic Analysis
Molecular testing of the F8 and F9 genes is used for carrier identification (females), prenatal diagnosis, and in cases of suspected inhibitor risk, particularly large inversions in the F8 gene.
Monitoring for Inhibitor Development (Complication)
The most significant complication of treatment is the patient developing an inhibitor—an alloantibody that neutralizes the infused replacement clotting factor. This requires specific monitoring.
- Bethesda Assay (or Nijmegen-Bethesda Assay): This assay detects and quantifies the concentration of anti-factor antibodies (inhibitors). Results are reported in Bethesda Units (BU). One BU is the amount of inhibitor that neutralizes 50% of the factor activity in a standard plasma sample after a two-hour incubation period. Detection of inhibitors changes the entire treatment plan, often requiring the use of bypassing agents instead of standard factor replacement. Inhibitors are more common in severe hemophilia, particularly Hemophilia A.
Summary of Expected Laboratory Results
| Test | Hemophilia A or B | Rationale |
|---|---|---|
| aPTT | Prolonged | Measures the Intrinsic Pathway (FVIII, FIX). |
| PT/TT | Normal | Extrinsic and final Common Pathways are intact. |
| Platelet Count | Normal | Primary hemostasis is intact. |
| Factor VIII Assay | Low (if Hemophilia A) | Confirms Type A and severity. |
| Factor IX Assay | Low (if Hemophilia B) | Confirms Type B and severity. |
Treatment and Management of Hemophilia
The goal of hemophilia management is to prevent bleeding episodes (prophylaxis) and to rapidly treat acute bleeding (on-demand therapy) to prevent long-term damage, particularly to the joints.
Replacement Therapy: The Mainstay of Treatment
The primary treatment involves replacing the deficient clotting factor (Factor VIII for Hemophilia A, Factor IX for Hemophilia B).
Factor Products
- Recombinant Factors (Preferred): These are produced using genetically engineered cell lines and are now the standard of care. They carry virtually no risk of transmitting blood-borne diseases.
- Standard Half-Life (SHL): Requires frequent infusions (3x weekly for FVIII; 2x weekly for FIX).
- Extended Half-Life (EHL): Modified factors that prolong the drug’s circulation time, allowing for less frequent infusions (e.g., once weekly or less), improving patient compliance and quality of life.
- Plasma-Derived Factors: Products purified from pooled human plasma. While viral inactivation and removal procedures make them safe, recombinant products are generally preferred.
Treatment Strategies
- Prophylaxis (Prevention): The standard of care for patients with severe hemophilia and often initiated in early childhood (Primary Prophylaxis) to prevent the first bleed and subsequent joint damage. The goal is to maintain factor levels above 1% to convert the clinical phenotype from severe to moderate/mild.
- Secondary Prophylaxis: Initiated after recurrent bleeds have already occurred.
- On-Demand Treatment: Administering factor concentrate only when a bleeding episode occurs or before a surgical/invasive procedure. This strategy is typically reserved for patients with mild or moderate hemophilia. Treatment must be initiated immediately upon suspicion of a bleed, especially in joints or the head.
Non-Factor Treatments and Adjunctive Therapies
These therapies are used alongside or instead of traditional factor replacement, depending on the patient’s condition and factor type.
- Desmopressin (DDAVP): DDAVP is a synthetic analog of vasopressin. It increases plasma levels of Factor VIII and von Willebrand factor (vWF) by triggering their release from storage sites within endothelial cells. It is highly effective and the first-line treatment for most patients with mild Hemophilia A. It is not effective in Hemophilia B or severe Hemophilia A.
- Antifibrinolytics: They stabilize the existing clot by inhibiting the breakdown of fibrin (fibrinolysis). They are excellent for mucosal bleeding (oral, dental, epistaxis) and often used as an adjunct during dental work or minor surgery. They should never be used alone for joint or muscle bleeds. For example, Tranexamic Acid (TXA) and Epsilon-Aminocaproic Acid (EACA).
- Emerging Therapies:
- Emicizumab (Hemlibra): A groundbreaking, non-factor therapy (approved for Hemophilia A). It is a bispecific monoclonal antibody that bridges Factor IXa and Factor X, essentially mimicking the function of missing Factor VIII. It uses subcutaneous administration (not IV) and less frequent dosing (weekly to monthly), significantly improving quality of life, and is highly effective for patients with inhibitors.
- Antithrombin (AT) Targeted Agents (e.g., Fitusiran): Small interfering RNA (siRNA) that inhibits the production of AT, a major natural anticoagulant. Lowering AT allows for more free thrombin to be generated, thus boosting hemostasis.
- Tissue Factor Pathway Inhibitor (TFPI) Inhibitors (e.g., Concizumab, Marstacimab): TFPI is a natural inhibitor of the extrinsic pathway. Blocking it enhances the initiation and propagation of coagulation.
- Gene Therapy: A potential cure aiming to introduce a functional copy of the FVIII or FIX gene into liver cells using a viral vector (often AAV). The liver then continuously produces the missing factor.
Joint Care and Rehabilitation
Since musculoskeletal damage (hemophilic arthropathy) is the primary long-term complication, management focuses heavily on joint health.
- Orthopedic Intervention: For end-stage arthropathy, procedures like synovectomy (removal of the inflamed joint lining) or total joint replacement may be necessary to relieve chronic pain and improve function.
- Acute Hemarthrosis Management: Factor infusion must be immediate, followed by the RICE principle:
- Rest: Immobilization of the affected joint.
- Ice: To reduce swelling and pain.
- Compression: Gentle support.
- Elevation: To reduce blood flow and swelling.
- Physical Therapy: Crucial for restoring joint range of motion and strengthening surrounding muscles after a bleed resolves. Physical therapy is also a cornerstone of long-term care to maintain joint health and prevent atrophy.
Hemophilia C: Factor XI Deficiency
Hemophilia C, also known as Factor XI (FXI) Deficiency, is distinct from Hemophilia A and B in its inheritance pattern, clinical presentation, and overall severity. It is characterized by its autosomal inheritance and a bleeding risk primarily associated with trauma or surgery, rather than spontaneous, debilitating joint bleeds.
| Feature | Hemophilia A (FVIII Deficiency) | Hemophilia B (FIX Deficiency) | Hemophilia C (FXI Deficiency) |
| Deficient Factor | Factor VIII (FVIII) | Factor IX (FIX) | Factor XI (FXI) (level often does not predict bleeding) |
| Inheritance | X-Linked Recessive | X-Linked Recessive | Autosomal Recessive |
| Bleeding Pattern | Severe spontaneous bleeding, notably hemarthrosis (joint bleeding) and muscle hematomas. | Severe spontaneous bleeding, notably hemarthrosis and muscle hematomas. | Variable; primarily post-traumatic or post-surgical (e.g., mucosal/dental bleeding). Spontaneous hemarthrosis is rare. |
| Standard Prophylaxis | Factor VIII Concentrates (SHL, EHL) or Emicizumab (Non-factor replacement). Prophylaxis is the standard of care for severe disease. | Factor IX Concentrates (SHL, EHL). Prophylaxis is the standard of care for severe disease. | Generally NOT required for spontaneous bleeding prevention. |
| Acute Bleeding Treatment | Immediate IV infusion of FVIII concentrate. | Immediate IV infusion of FIX concentrate. | Antifibrinolytics (e.g., tranexamic acid) are often sufficient. FXI concentrate or FFP for major procedures/bleeds. |
| Inhibitor Management | High risk (~ 20-30% severe HA). Managed with Bypassing Agents (aPCC, rFVIIa) or Immune Tolerance Induction (ITI). | Low risk (~ 3-5% severe HB). Managed with Bypassing Agents or ITI. | Extremely Rare. Not a major clinical concern. |
| Novel Therapies | Emicizumab, Gene Therapy (FVIII). | Gene Therapy (FIX). | Currently no approved novel therapies due to milder/different clinical needs. |
Frequently Asked Questions (FAQs)
Can a person with hemophilia live a normal life?
Yes, a person with hemophilia can live a normal life. With proper treatment and management, individuals with hemophilia can enjoy many of the same activities and experiences as anyone else.
Advances in medical technology, particularly in the area of blood clotting factor replacement therapy, have significantly improved the quality of life for people with hemophilia. This treatment involves replacing the missing clotting factor in the blood, allowing for better blood clotting and reducing the risk of bleeding episodes.
While there may be certain precautions to take, such as avoiding contact sports or activities that could lead to injuries, individuals with hemophilia can still engage in a wide range of activities, including education, employment, and hobbies.
It’s important to note that the severity of hemophilia can vary, and individual experiences may differ. However, with appropriate care and support, people with hemophilia can lead fulfilling and active lives.
Is hemophilia life threatening?
Hemophilia can be life-threatening if not managed properly. While advancements in treatment have significantly improved the quality of life for individuals with hemophilia, uncontrolled bleeding can lead to serious complications, including:
- Internal bleeding: This can occur in organs like the brain, joints, or muscles, leading to pain, swelling, and even organ damage.
- Hemarthrosis: This is bleeding into the joints, which can cause pain, swelling, and decreased mobility.
- Spontaneous bleeding: This can occur without any apparent injury or trauma.
- Life-threatening bleeding: In severe cases, uncontrolled bleeding can lead to shock or death.
However, with proper treatment, including regular monitoring, medication, and preventative measures, individuals with hemophilia can significantly reduce the risk of serious complications and live long, healthy lives.
Can hemophilia get worse with age?
Yes, hemophilia can get worse with age. While the severity of hemophilia varies from person to person, it is generally considered a lifelong condition. As people age, their bodies may undergo changes that can affect the effectiveness of clotting factor replacement therapy, leading to an increased risk of bleeding episodes.
Additionally, some individuals with hemophilia may develop inhibitors, which are antibodies that interfere with the effectiveness of clotting factor replacement therapy. These inhibitors can make it more difficult to manage hemophilia and may require specialized treatments.
Can hemophiliacs donate blood?
No, individuals with hemophilia cannot donate blood. People with hemophilia often rely on blood products, such as clotting factor concentrates, to manage their condition. Donating blood would reduce the available supply of these essential products.
Is hemophilia a painful condition?
Yes, hemophilia can be a painful condition. Bleeding episodes, particularly into the joints, can cause significant pain, swelling, and stiffness. These symptoms can interfere with daily activities and reduce quality of life.
While advancements in treatment have significantly improved the management of hemophilia, individuals with the condition may still experience pain, especially during periods of active bleeding or joint damage. It’s important to seek prompt medical attention for any pain or discomfort associated with hemophilia.
Why is the APTT prolonged in hemophilia while the PT is normal?
The PT measures the extrinsic pathway (initiated by Factor VII and Tissue Factor), which is unaffected by FVIII or FIX deficiency. The APTT measures the intrinsic and common pathways. Since FVIII and FIX are critical components of the intrinsic pathway’s tenase complex, their deficiency results in a significantly prolonged APTT.
What is the primary difference in inhibitor development between Hemophilia A and B?
Inhibitor development is significantly more common in severe Hemophilia A (~20-30%) than in severe Hemophilia B (~3-5%). Furthermore, inhibitors in Hemophilia B can, in rare cases, be associated with severe allergic or anaphylactic reactions due to immune response against the infused FIX.
When should a physician consider transitioning a Hemophilia A patient to Emicizumab prophylaxis?
Emicizumab is a revolutionary prophylactic treatment primarily indicated for Hemophilia A patients (with or without inhibitors) who have a high treatment burden, poor venous access, or high adherence challenges with IV factor infusions. It is a highly effective, non-factor replacement option that dramatically reduces bleeding rates and improves quality of life.
What is the rationale behind Extended Half-Life (EHL) factors, and are they available for both FVIII and FIX?
The goal of EHL factors is to reduce the frequency of intravenous infusions required for prophylaxis. EHL factors are available for both FVIII and FIX, often through modification (e.g., Fc fusion or PEGylation). The half-life extension is generally more pronounced for FIX (allowing weekly or bi-weekly dosing) than for FVIII.
Glossary of Medical Terms
- Hemarthrosis: Bleeding into a joint space, a defining clinical feature of severe hemophilia.
- Prophylaxis: The regular, scheduled infusion of factor concentrate (or non-factor agent) to prevent bleeding episodes.
- Inhibitor: An alloantibody (IgG) developed by the immune system against the infused therapeutic factor concentrate, rendering replacement therapy ineffective.
- Bethesda Unit (BU): The unit of measure used to quantify the concentration of a factor inhibitor in a patient’s plasma.
- Tenase Complex: The enzymatic complex formed by FIXa and its cofactor FVIIIa, responsible for activating Factor X in the intrinsic coagulation pathway.
- Emicizumab: A bispecific antibody (non-factor therapy) that mimics the bridging function of FVIIIa to link FIXa and FX.
- Hemophilic Arthropathy: Chronic, degenerative joint disease resulting from recurrent hemarthrosis and subsequent inflammatory changes in the synovium and cartilage.
- Immune Tolerance Induction (ITI): A resource-intensive therapeutic strategy involving high-dose factor infusion aimed at eliminating or neutralizing inhibitors.
- Extended Half-Life (EHL) Factors: Modified recombinant factors (FVIII or FIX) engineered to prolong their circulation time in the bloodstream, allowing for less frequent infusions.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Saba HI, Roberts HR. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management (Wiley Blackwell). 2014.
- DeLoughery TG. Hemostasis and Thrombosis 4th Edition (Springer). 2019.
- Keohane EM, Otto CN, Walenga JM. Rodak’s Hematology 6th Edition (Saunders). 2019.
- Tiede A, Collins P, Knoebl P, Teitel J, Kessler C, Shima M, Di Minno G, d’Oiron R, Salaj P, Jiménez-Yuste V, Huth-Kühne A, Giangrande P (2020). International recommendations on the diagnosis and treatment of acquired hemophilia A. Haematologica; 105(7). https://doi.org/10.3324/haematol.2019.230771.
- Leebeek FWG, Miesbach W. Gene therapy for hemophilia: a review on clinical benefit, limitations, and remaining issues. Blood (2021) 138 (11): 923–931.
- Berntorp, E., Fischer, K., Hart, D.P. et al. Haemophilia. Nat Rev Dis Primers 7, 45 (2021). https://doi.org/10.1038/s41572-021-00278-x
- Sarmiento Doncel S, Díaz Mosquera GA, Cortes JM, Agudelo Rico C, Meza Cadavid FJ, Peláez RG. Haemophilia A: A Review of Clinical Manifestations, Treatment, Mutations, and the Development of Inhibitors. Hematol Rep. 2023 Feb 16;15(1):130-150. doi: 10.3390/hematolrep15010014. PMID: 36810557; PMCID: PMC9944491.
- Mehta P, Reddivari AKR. Hemophilia. [Updated 2023 Jun 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK551607/
- Young G, Lassila R, Mason J, Prasca S. Deconstructing the ISTH hemophilia guidelines for the clinician. J Thromb Haemost. 2025 May;23(5):1483-1495. doi: 10.1016/j.jtha.2024.11.015. Epub 2024 Nov 29. PMID: 39617189.
- Kruse-Jarres R, Kempton CL, Baudo F, Collins PW, Knoebl P, Leissinger CA, Tiede A, Kessler CM. Acquired hemophilia A: Updated review of evidence and treatment guidance. Am J Hematol. 2017 Jul;92(7):695-705. doi: 10.1002/ajh.24777. Epub 2017 Jun 5. PMID: 28470674.
- Batorova A, Boban A, Brinza M, Lissitchkov T, Nemes L, Zupan Preložnik I, Smejkal P, Zozulya N, Windyga J. Expert opinion on current and future prophylaxis therapies aimed at improving protection for people with hemophilia A. J Med Life. 2022 Apr;15(4):570-578. doi: 10.25122/jml-2022-0103. PMID: 35646171; PMCID: PMC9126455.