Procedure-at-a-Glance
| Step | Action |
| 1. Preparation | Prepare 1:1 mix of Patient Plasma (PP) and Pooled Normal Plasma (NP). |
| 2. Immediate Mix | Run APTT on NP, PP, and the 1:1 Mix immediately. |
| 3. Incubation | Incubate the 1:1 Mix and NP at 37°C for 1–2 hours. |
| 4. Post-Incub. Run | Run APTT on the incubated tubes. |
| 5. Interpretation | Compare results against the “Correction” threshold. |
Introduction
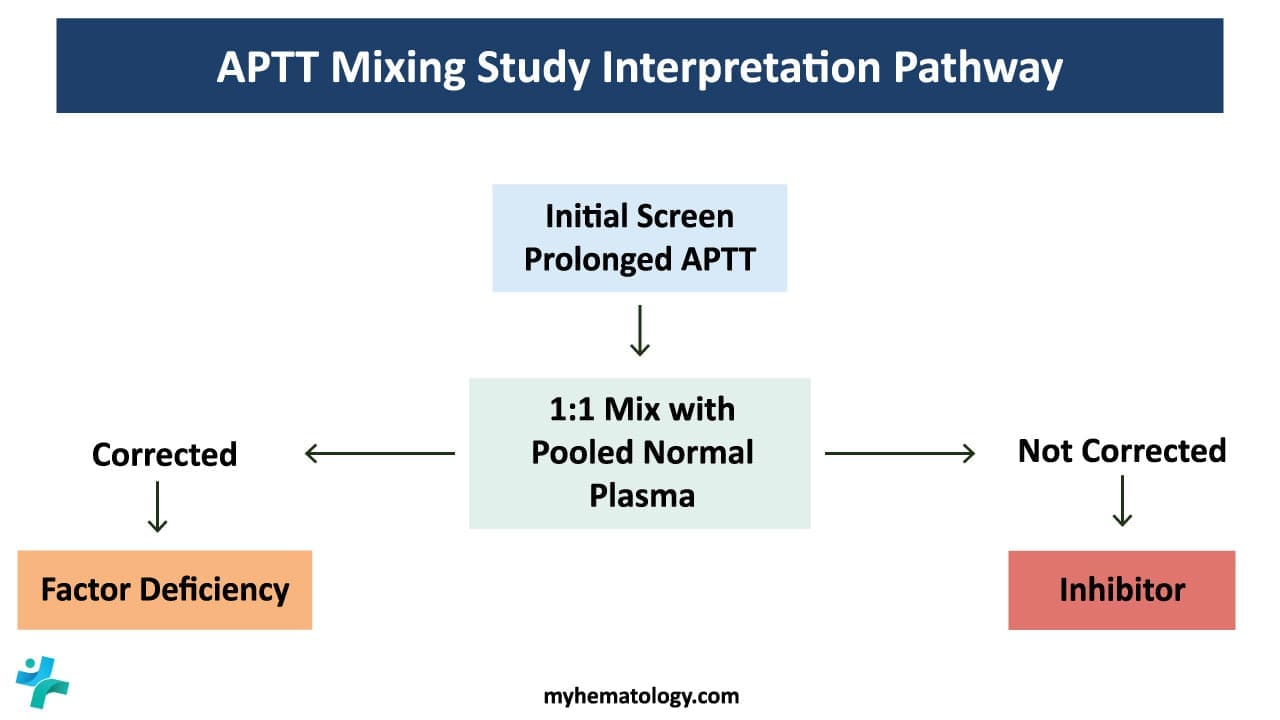
The activated partial thromboplastin time (APTT) is a coagulation test that measures the intrinsic pathway of blood clotting. While the APTT itself is a valuable diagnostic tool, it can be further enhanced through the use of manual mixing study. A mixing study is carried out when there is an isolated prolongation of APTT. The mixing study helps differentiate between factor deficiencies and the presence of inhibitors towards coagulation factors, providing crucial information for the diagnosis and management of bleeding disorders.
For example, distinguishing a Lupus Anticoagulant (LA) from a Specific Factor Inhibitor (like an anti-Factor VIII antibody) is important in a mixing study. While both result in a “failure to correct,” their clinical implications are opposites: LA is associated with thrombosis (clotting), while factor inhibitors are associated with hemorrhage (bleeding).
The primary difference lies in what the inhibitor is attacking.
- Specific Factor Inhibitors: These are antibodies (usually IgG) that target a specific coagulation protein (e.g., Factor VIII). No matter how much phospholipid you add, the factor remains neutralized.
- Lupus Anticoagulants: These are heterogeneous antibodies that bind to phospholipid-protein complexes. Because the APTT test relies on phospholipids to trigger the “contact” pathway, the LA interferes with the reagent’s ability to facilitate clotting.
The “Paradox” of Lupus Anticoagulant
- In the Test Tube (In Vitro): LA acts as an anticoagulant. It interferes with the phospholipids in the reagent, causing the APTT to look “long,” as if the blood is thin.
- In the Human Body (In Vivo): LA acts as a procoagulant. It is strongly associated with Antiphospholipid Syndrome (APS), leading to venous or arterial clots and pregnancy complications.
Principle of Mixing Study
A manual mixing study involves mixing the patient’s plasma with different reagents and observing the resulting APTT. The specific reagents used depend on the suspected cause of the prolonged APTT. Here are some common approaches:
- 1:1 Mixing with normal plasma: This mixing study mixes the patient’s plasma 1:1 with normal plasma. If the APTT corrects to the normal range, it suggests a factor deficiency. If the APTT remains prolonged after the mixing study, it indicates the presence of an inhibitor.
- Mixing with specific factor concentrates: This mixing study involves mixing the patient’s plasma with individual factor concentrates to identify the specific factor deficiency.
- Mixing with phospholipid reagents: This mixing study helps assess the function of phospholipids, which are essential for the intrinsic pathway.
Materials
- #Platelet poor plasma (PPP) of patient. PPP is prepared by centrifuging the peripheral blood at 2000 g for 15 minutes at room temperature.
- Commercially available normal plasma pool
- *Activator-phospholipid solution
- *0.025 mol/L calcium chloride (CaCl2)
- *Glass tubes 5 ml
- *Water bath
- Ice bath or crushed-ice
- Stopwatch
- Timer
- Pipettes 100 – 200 ul
- Pipette tips
*These items must be at 37°C.
#PPP must be kept at room temperature to prevent activation of factor VIII which may cause an inaccurate timing result.
Protocol
Tube Preparation
- Label four tubes:
- Tube 1: Normal plasma (NP)
- Tube 2: Patient plasma (PP)
- Tube 3: Mixed plasma (MP)
- Tube 4: Incubated mixed plasma (IMP)*
- Dispense:
- Tube 1: 0.5 mL NP
- Tube 2: 0.5 mL PP
- Tube 3: 0.25 mL NP + 0.25 mL PP
- Tube 4: 0.25 mL NP + 0.25 mL PP
APTT Measurement
- Perform APTT in duplicate for tubes 1, 2, and 3 following a normal APTT protocol.
- Add 1 mL of activator-phospholipid solution into each test tube and immediately start the timer.
- Incubate the tubes in the water bath for 3 minutes with occasional gentle agitation.
- Get the stopwatch ready, add 0.5 mL CaCl2 and immediately start the stopwatch.
- Gently mix the solution in the test tubes by tilting the test tubes to 45° angle and agitate lightly.
- Intermittently take the tubes out of the water bath to observe for the first sign of clot formation.
- Stop the timer and record the time at the first sign of clot formation.
- Incubation: Incubate tube 4 at 37°C for 120 minutes.
- Cooling: After incubation, transfer tube 4 to an ice bath or crushed ice.
- Final APTT: Perform APTT on the incubated mixed plasma (tube 4) following the previously described steps 2 – 7.
*Incubated mixed plasma is to check for time-dependent inhibitors.
How to Interpret APTT Mixing Study
Based on the results of the mixing study, the clinician can draw conclusions about the cause of the prolonged APTT:
- Corrected APTT: A corrected APTT after mixing with normal plasma or specific factor concentrates indicates a factor deficiency. The specific factor deficiency can be identified by further analysis or by observing the correction pattern with different factor concentrates.
- Uncorrected APTT: An uncorrected APTT suggests the presence of an inhibitor. Further tests are needed to identify the specific inhibitor and its cause.
Mixing with Normal Plasma
- 1:1 Mixing: This is the most common approach. Equal volumes of patient plasma and normal control plasma are mixed.
- Interpretation:
- If the APTT corrects (within the normal reference interval), it suggests a factor deficiency. The normal plasma replenishes the deficient factor, leading to normal clotting.
- If the APTT remains prolonged, it indicates an inhibitor, likely lupus anticoagulants (LA), which is not neutralized by the added normal plasma.
Interpretation of APTT Mixing Study Results
| Immediate Mix (1:1) | Incubated Mix (37°C / 2hrs) | Likely Interpretation | Clinical Significance |
| Corrects | Corrects | Factor Deficiency | Indicates a deficiency in Factors VIII, IX, XI, or XII. The normal plasma supplied the missing factor. |
| No Correction | No Correction | Immediate Inhibitor | Suggests a strong inhibitor, most commonly Lupus Anticoagulant (LA) or a high-titer specific factor inhibitor. |
| Corrects | No Correction (Prolongs again) | Time-Dependent Inhibitor | Classically indicates a Factor VIII Inhibitor (Acquired Hemophilia A). The inhibitor needs time/warmth to neutralize the factor. |
| No Correction | Corrects | Pre-analytical Error | Rare and usually invalid; suggests an issue with the incubation process or reagent degradation. Should be repeated. |
Mixing with Specific Factor Concentrates
- Individual factor concentrates: This approach is used when the specific factor deficiency is known or suspected. Patient plasma is mixed with a specific factor concentrate to correct the deficiency.
- Interpretation:
- If the APTT corrects after mixing with the specific factor concentrate, it confirms the factor deficiency.
- If the APTT remains prolonged, it suggests the presence of an inhibitor along with the factor deficiency.
Mixing with Phospholipid Reagents (Phospholipid Neutralization Test)
LA is an antibody that targets the phospholipids used in the APTT reagent, adding a massive “excess” of phospholipids essentially distracts the inhibitor. If the clotting time shortens significantly after adding phospholipids, you have a confirmed LA.
- LA-sensitive reagents: Some phospholipid reagents are specifically sensitive to LA. Mixing patient plasma with these reagents can help detect and differentiate LA from other inhibitors.
- Interpretation:
- If the APTT corrects after mixing with an LA-sensitive reagent, it suggests the presence of LA.
- If the APTT remains prolonged, it indicates a different type of inhibitor or another underlying cause for the prolonged APTT.
While the Phospholipid Neutralization Test (PNT) is a classic and highly effective confirmatory test for Lupus Anticoagulant, it is best used as part of a multi-test profile. Most experts consider the combination of the dRVVT and the APTT-PNT as the diagnostic gold standard, as no single test can detect 100% of Lupus Anticoagulants.
Advanced Interpretation: Mixing with Concentrates and Phospholipids
| Result Scenario | Immediate Mix (1:1) | + Specific Factor Concentrate | + Excess Phospholipids | Final Interpretation |
| Factor Deficiency | Corrects | Corrects (with specific factor) | No significant change | Identifies which factor is missing (e.g., IX vs. XI). |
| Lupus Anticoagulant (LA) | No Correction | No significant change | Corrects | Confirms LA. The excess phospholipid “soaks up” the inhibitor, allowing the test to normalize. |
| Specific Factor Inhibitor | No Correction | No Correction | No significant change | Confirms a Specific Inhibitor (e.g., Anti-Factor VIII). The inhibitor neutralizes even the added concentrate. |
| Heparin Contamination | No Correction | No significant change | No significant change* | Heparin Effect. (Note: Heparin is neutralized by protamine or heparinase, not usually phospholipids). |
Additional Approaches
- Serial dilutions: This involves mixing the patient’s plasma with varying dilutions of normal plasma or specific factor concentrates. The purpose is to determine the strength of the inhibitor.
- Incubation: Incubating the mixture of patient plasma and normal plasma or specific factor concentrates before performing the APTT can sometimes neutralize certain inhibitors, aiding in diagnosis.
Rosner Index & Percent Correction
The Rosner Index and Percent Correction is important as these calculations move the interpretation from subjective (“it looks corrected”) to objective, data-driven results.
The Rosner Index (Index of Circulating Anticoagulant)
The Rosner Index is the most widely used formula to determine if a mix has “corrected.” It specifically highlights the presence of an inhibitor by calculating the gap between the mix and the normal plasma, relative to the patient’s initial prolongation.
The Formula
Rosner Index = (1:1 Mix APTT – NP APTT)/(PP APTT) X 100
- PP: Patient Plasma APTT
- NP: Normal Plasma APTT
- 1:1 Mix: The result of the mixed sample
Interpretation
- < 12: Indicates Correction (Factor Deficiency).
- 12 – 15: Borderline (Gray zone; usually requires further testing or clinical correlation).
- > 15: Indicates No Correction (Presence of an Inhibitor/Lupus Anticoagulant).
Percent (%) Correction
Some laboratories prefer the Percent Correction method. This calculation measures how much of the “prolongation” was fixed by adding normal plasma.
The Formula
% Correction = (PP APTT – 1:1 Mix APTT)/(PP APTT – NP APTT) X 100
Interpretation
- > 70%: Indicates Correction (Factor Deficiency).
- < 50%: Indicates No Correction (Inhibitor).
- 50% – 70%: Borderline.
Choosing the Right Approach
The choice of mixing study approach depends on several factors, including:
- Clinical presentation: The patient’s symptoms and medical history may suggest a specific type of factor deficiency or inhibitor.
- Initial APTT result: The degree of APTT prolongation can guide the selection of appropriate mixing studies.
- Availability of resources: Some laboratories may not have access to all reagents or equipment necessary for specific mixing studies.
Advantages of Manual Mixing Study
A manual mixing study offer several advantages over other methods:
- Simplicity and cost-effectiveness: The mixing study require minimal resources and can be easily performed in most laboratories.
- Rapid results: Mixing study results are available within minutes, allowing for prompt diagnosis and treatment decisions.
- Flexibility: Different mixing ratios and reagents can be used in the mixing study depending on the clinical context.
Limitations of Manual Mixing Study
Despite their advantages, a manual mixing study have some limitations:
- Subjectivity: Interpretation of mixing study results can be subjective and may depend on the experience of the laboratory personnel.
- Limited sensitivity: The mixing study might not detect low levels of inhibitors or factor deficiencies.
- Standardization: Lack of standardized procedures can lead to variations in mixing study results between different laboratories.
Troubleshooting
Pre-analytical Errors
These occur before the sample even reaches the analyzer. If these aren’t controlled, the mixing study results could be clinically dangerously misleading.
- Heparin Contamination: The most common “imposter.” Even a tiny amount of heparin from a line flush will cause a failure to correct, mimicking a powerful inhibitor.
- Inadequate Centrifugation (Residual Platelets): If the sample is not “Platelet-Poor Plasma” (PPP) (< 10 x 109/L), the residual platelets will break open during freezing or incubation. They release Platelet Factor 4 (PF4) and phospholipids, which can neutralize a Lupus Anticoagulant, leading to a false-negative result.
- The “Hematocrit Effect”: If a patient has an extremely high hematocrit (>55%), the volume of plasma in the collection tube is reduced. This leads to an over-concentration of the sodium citrate anticoagulant, which binds all the calcium in the test system and falsely prolongs the APTT.
- Sample Age and Temperature: Coagulation factors (especially Factor VIII) are “labile.” If the sample sits at room temperature for too long, the factors degrade. This can lead to a prolonged baseline APTT that has nothing to do with a clinical deficiency.
Other Causes
| Observation | Potential Cause | Action/Resolution |
| Normal Plasma (NP) prolongs after incubation. | Reagent/Plasma degradation. | Check water bath temp (must be 37°C). Use fresh NP aliquot. |
| Immediate Mix corrects, but Incubated Mix fails. | Time-dependent inhibitor (FVIII). | Perform Bethesda Assay to quantify the inhibitor. |
| Unexpectedly high Rosner Index in a healthy patient. | Heparin contamination or high Hematocrit. | Run Thrombin Time or check the tube fill volume. |
| Mix corrects, but Factor Assay is still low. | “Lupus Anticoagulant” interference. | LA can sometimes interfere with factor assays; use chromogenic assays instead. |
Frequently Asked Questions (FAQs)
Why must the 1:1 mix be incubated?
Some inhibitors, specifically Factor VIII antibodies (found in Acquired Hemophilia A), are “time-dependent.” They do not neutralize the factor immediately; therefore, the “Immediate Mix” might look like a correction, while the “Incubated Mix” will show prolongation.
What is the Rosner Index?
It is a mathematical formula used to standardize the interpretation of mixing studies. A Rosner Index of <12 typically indicates correction (deficiency), while >15 indicates a failure to correct (inhibitor).
Can heparin interfere with a mixing study?
Yes. Heparin in the patient sample will prolong both the patient’s APTT and the 1:1 mix, mimicking an inhibitor. To rule this out, a Thrombin Time (TT) or a Heparinase neutralization test should be performed.
Why do we use “Platelet-Poor Plasma” (PPP) for this test?
Platelets contain phospholipids that can neutralize Lupus Anticoagulants. If the plasma is not properly centrifuged to remove platelets, you may get a “false correction” in a patient who actually has an inhibitor.
Glossary of Related Medical Terms
- Pooled Normal Plasma (PNP): A mixture of plasma from at least 20 healthy donors used to represent 100% factor activity.
- Inhibitor: An antibody (usually IgG) that interferes with coagulation factors, either by neutralizing them or increasing their clearance.
- Lupus Anticoagulant (LA): An antiphospholipid antibody that interferes with phospholipid-dependent tests, causing an artificial prolongation of APTT in vitro.
- Correction: When the APTT of a 1:1 mix falls within a specific range (usually within 10% or 5 seconds) of the normal range, indicating the NP supplied the missing factors.
- Factor VIII Inhibitor: A specific autoantibody that often requires incubation time to show its full inhibitory effect (time-dependent).
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- Bain BJ, Bates I, Laffan MA. Dacie and Lewis Practical Haematology: Expert Consult: Online and Print 12th Edition (Elsevier). 2016.
- Saba HI, Roberts HR. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management 1st Edition (Wiley-Blackwell). 2014.
- Favaloro E. J. (2020). Coagulation mixing studies: Utility, algorithmic strategies and limitations for lupus anticoagulant testing or follow up of abnormal coagulation tests. American journal of hematology, 95(1), 117–128. https://doi.org/10.1002/ajh.25669
- Adcock, D. M., Moore, G. W., Kershaw, G. W., Montalvao, S. A. L., & Gosselin, R. C. (2024). International Council for Standardization in Haematology (ICSH) recommendations for the performance and interpretation of activated partial thromboplastin time and prothrombin time mixing tests. International journal of laboratory hematology, 46(5), 777–788. https://doi.org/10.1111/ijlh.14344