Introduction
Unraveling the mysteries of life often requires peering into the microscopic world of DNA. Conventional Polymerase Chain Reaction (PCR) is a powerful technique that allows us to amplify specific DNA sequences, making them visible and easily analyzed for example in diagnosing specific beta-thalassemia mutations. We can use agarose gel to make sure that the right amplicons have been amplified before sending the PCR product for sequencing. This protocol focuses on the final step of this process: agarose gel electrophoresis, where we separate and visualize the amplified DNA fragments.
Imagine a tiny copy machine for DNA! PCR takes a target DNA sequence and replicates it millions of times through repeated cycles of heating, annealing and cooling. Specific primers flank the target region, guiding the DNA polymerase enzyme to faithfully copy the desired stretch. This exponential amplification allows us to detect even minute amounts of DNA, making PCR invaluable for various applications in medicine, research, and forensics.
But how do we see these amplified DNA fragments? This is where agarose gel electrophoresis comes in. Molten agarose, a seaweed-derived polymer, solidifies into a mesh-like network when cooled. When an electric field is applied, negatively charged DNA molecules migrate through the gel pores at different rates depending on their size. Smaller fragments move faster and travel farther, while larger ones are hindered by the gel matrix.
The gel is stained with a fluorescent nucleic acid dye like ethidium bromide (EtBr) or SYBR safe stains or RedSafeTM stain. These stains bind to the PCR amplicons and fluoresce when excited using ultraviolet (UV) light. Thus, allowing us to visualize these separated DNA bands. Each band represents a specific amplified DNA fragment, providing valuable information about the presence, size, and abundance of the target sequence in the original sample.
This protocol will guide you through the steps of preparing an agarose gel for conventional PCR analysis.
Principle
The foundation lies in the unique properties of agarose, a natural polymer extracted from seaweed. When heated, agarose forms a semi-solid, jelly-like matrix, riddled with tiny pores. These pores, like miniature tunnels, act as a sieving mechanism for molecules, especially charged ones like DNA.
DNA molecules, being negatively charged due to their phosphate backbone, experience an attractive force towards the positive electrode when an electric field is applied across the gel. This electrophoretic pull sets them on a journey through the agarose gel.
But not all DNA molecules navigate the gel with equal ease. This differential mobility, dictated by size, is the key to agarose gel’s use. Smaller DNA fragments, due to their compact structure, can readily wiggle through the narrow pores, encountering less resistance and zipping towards the positive end. Larger fragments, on the other hand, face a tougher obstacle course. Their bulkier size makes them snag on the agarose strands, slowing down their progress.
Materials
For Agarose Gel
- Agarose powder
- 1x TBE or TAE buffer (Tris-Acetate-EDTA or Tris-Acetate-Borate buffer)
- Microwave
- Erlenmeyer flask or microwave-safe flask (e.g., 250 mL)
- Gel casting tray with comb
- Staining dye (e.g., ethidium bromide)
- Gloves
- Safety goggles
For Sample Loading
- Micropipettes and pipette tips
- DNA samples (PCR products, plasmids, etc.)
- DNA loading dye
- DNA ladder (e.g., 100 bp DNA ladder)
Protocol
Preparation
- Wear gloves and safety goggles throughout the procedure.
- Weigh out the desired amount of agarose powder (e.g., 1 gram for a 1% gel in 100 mL buffer). Usually 1.5 – 2% gel is used. However, if the amplicons designed are very close together in size, 3% gel can be used.
- Add the agarose powder to the Erlenmeyer flask or microwave-safe flask.
- Pour in the TBE or TAE buffer until the desired volume is reached (e.g., 100 mL for a 1% gel). For less than 10 samples, a small gel of 30 mL can be made.
- Swirl gently to evenly distribute the agarose powder.
Melting
- Microwave the agarose/buffer mixture on high power for 30 – 60 seconds intervals, swirling the flask gently after each interval, until the agarose is completely dissolved and the solution is clear. The agarose gel is completely dissolved when there are no longer small, tiny bubbles in the solution. The solution should be clear without any bubbles when it settles.
- Be careful as the solution will be hot! Use heat-resistant gloves and handle the flask with caution.
Cooling and Pouring
- Assemble the tray, cassette and comb using the desired sizes of gel and number of wells.
- Add the desired amount of staining dye (e.g., 5 µL of ethidium bromide per 100 mL of agarose solution).
- Swirl gently to mix the dye.
- Carefully pour the warm agarose solution into the gel casting tray, ensuring the wells are oriented correctly. Ensure that there are no bubbles in the gel. Use a pipette tip to pop any bubbles or to push the bubbles to the side of the gel away from the path of wells.
- Allow the gel to solidify completely at room temperature for 30-45 minutes or until it’s firm. The gels can be prepared in advance, wrapped in a cling film once solidified and placed in the fridge until ready to use. Do not prepare too far in advance as the gel may dry up if left too long in the fridge.
Sample Loading and Running
- Once the gel is solidified, carefully remove the comb.
- Place the gel cassette in the electrophoresis tank and fill it with enough buffer to cover the gel.
- Pipette your DNA samples (5 uL) including the non-template control, mixed with an equal amount of loading dye (if necessary), into the wells.
- Pipette the DNA ladder (3 uL), mixed with an equal amount of loading dye (if necessary) into one of the wells, preferably the first well.
- Close the lid of the tank and connect it to the power supply. Make sure that the top of the gel (where the samples are loaded) is on the negative side of the power supply as the DNA is negatively charged and will move towards the positive end.
- Run the electrophoresis at the desired voltage for the appropriate time (e.g., 70-100 V for 30-60 minutes).
Visualization
- After electrophoresis, transfer the gel to a UV transilluminator or gel documentation system and visualize the DNA bands or PCR amplicons.
- Take a picture or analyze the bands.
Interpretation
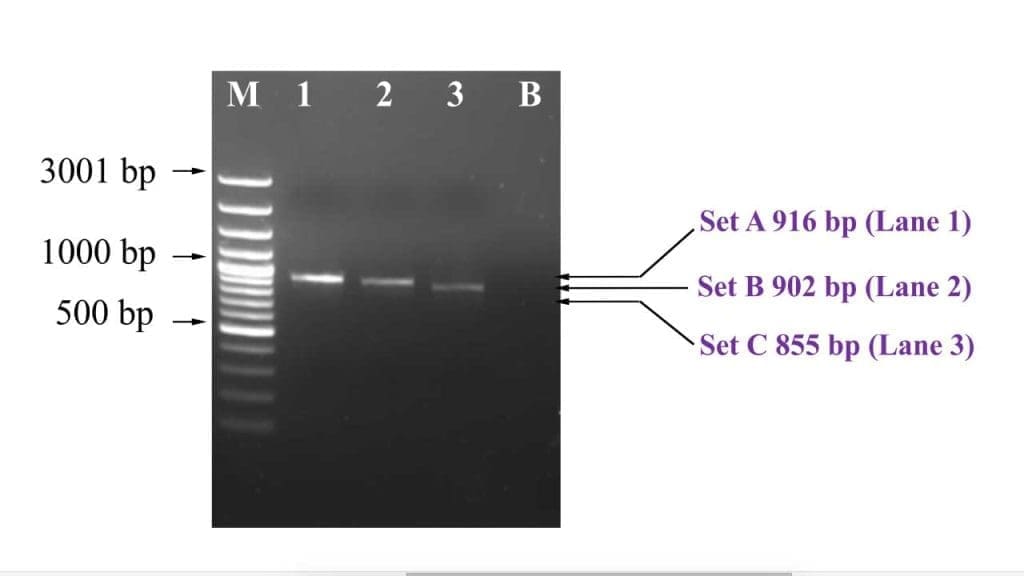
It is imperative to check that the right bands have been amplified especially using a DNA ladder. To be sure, the PCR amplicons can be sent for sequencing to ensure that the exact band that is required was correctly amplified. Non-template control is important as a negative control for contamination.
Using the right agarose concentration and the right well size goes a long way in reporting a PCR result.
Troubleshooting Tips for Agarose Gel Preparation
While agarose gel preparation seems straightforward, it can sometimes lead to frustration when things don’t go as planned. Here are some common issues you might encounter and tips to get your gel back on track:
1. Gel not solidifying
- Possible cause: Wrong agarose powder concentration calculations or incomplete agarose melting or or insufficient cooling.
- Solution: Make sure that the agarose powder used is in the right concentration. Microwave the agarose solution in short bursts, swirling in between, until completely dissolved. Ensure the solution cools to around 50°C before pouring.
2. Bubbles in the gel
- Possible cause: Trapped air during pouring.
- Solution: Swirl the agarose gently while pouring and avoid over-heating the solution. You can also try poking the bubbles with a sterile pipette tip or push the bubbles to the side of the gel.
3. Bands blurry or smeared
- Possible cause: Overloading the wells, high voltage, or denatured DNA.
- Solution: Load smaller sample volumes, adjust the voltage based on your fragment size, and ensure proper sample preparation to avoid denaturation.
4. Faint or no bands:
- Possible cause: Low DNA concentration, insufficient staining, or inadequate electrophoresis time.
- Solution: Quantify your DNA samples, increase staining intensity, or run the gel for a longer time depending on your fragment size.
5. Uneven band migration:
- Possible cause: Tilted gel, uneven buffer levels, or bubbles under the comb.
- Solution: Ensure the gel tray is level, fill the tank with enough buffer to cover the gel evenly, and remove any bubbles trapped under the comb before electrophoresis.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- https://www.addgene.org/protocols/gel-electrophoresis/
- R. Green MR, Sambrook J. Molecular Cloning: A Laboratory Manual (Fourth Edition), Volume 1, 2 & 3. Cold Spring Harbor Laboratory Press. 2012.
- Armstrong JA, Schulz JR. Agarose Gel Electrophoresis. 2015. Current Protocols: Essential Laboratory Techniques. https://doi.org/10.1002/9780470089941.et0702s10