TL;DR
Eosinophilic asthma is a severe, late-onset type of asthma characterized by a high number of eosinophils, which drives chronic airway inflammation that is often resistant to standard treatments and responds best to targeted biologic therapies.
- Symptoms and Signs ▾: Late-onset and more severe than other asthma types. Key symptoms include persistent wheezing, shortness of breath, chest tightness, and a chronic cough that is poorly responsive to standard inhaled steroids. It’s often associated with other conditions like chronic rhinosinusitis with nasal polyps.
- Diagnosis ▾: The diagnosis relies on confirming elevated eosinophil levels. The most definitive test is a sputum eosinophil count (typically >3%), but other important markers include a high blood eosinophil count (>300 cells/μL) and an elevated fractional exhaled nitric oxide (FeNO) level (>25 ppb), which indicates airway inflammation.
- Treatment ▾: Standard therapies like inhaled corticosteroids (ICS) and long-acting beta-agonists (LABA). For severe cases, the focus shifts to biologic therapies that target specific inflammatory pathways. These include anti-IL-5 agents (mepolizumab, reslizumab, benralizumab), anti-IL-4/IL-13 agents (dupilumab), and anti-TSLP agents (tezepelumab).
- Prognosis and Complications ▾: Targeted therapies can significantly improve a patient’s quality of life and reduce the frequency of exacerbations. However, if left untreated, the disease can lead to serious complications, including irreversible airway remodeling, frequent life-threatening exacerbations, and dependence on oral corticosteroids.
*Click ▾ for more information
Introduction
Eosinophilic asthma is a distinct and often severe phenotype of asthma characterized by a prominent inflammatory response driven by eosinophils. Unlike allergic asthma, which is typically triggered by specific allergens, eosinophilic asthma can be non-allergic and often develops in adulthood.
This condition is significant because it often doesn’t respond well to standard asthma treatments, and its diagnosis is key to guiding more targeted therapies.
Pathophysiology of Eosinophilic Asthma
The pathophysiology of eosinophilic asthma is centered on a Type 2 inflammatory response orchestrated by cytokines that promote the recruitment, maturation, and activation of eosinophils. This inflammatory cascade is fundamentally regulated by a crucial transcription factor, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which acts as a master switch for the expression of pro-inflammatory genes.
The Initiators: Th2 Cells and ILC2s
Eosinophils are granulocytes that play a key role in the immune system, particularly in defense against parasitic infections. In eosinophilic asthma, however, these cells become the primary drivers of pathology. Stimuli such as viral infections, environmental irritants, or other unidentified triggers lead to the activation of T helper type 2 (Th2) cells and Type 2 innate lymphoid cells (ILC2s) in the airways.
The stimulus binds to a receptor on the cell’s surface. This triggers a signaling cascade within the cell. This cascade leads to the activation of the NF-κB transcription factor, which is already present but inactive in the cell’s cytoplasm.
Once activated, NF-κB moves into the nucleus of that same cell and turns on the genes that produce pro-inflammatory cytokines primarily interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13).
The Role of Key Cytokines
- IL-4 and IL-13: These two cytokines work together to promote inflammation in the airways. They stimulate B cells to produce immunoglobulin E (IgE), which is a key player in allergic reactions. They also cause the airway smooth muscle to constrict and increase mucus production, leading to many of the classic asthma symptoms like wheezing and coughing.
- IL-5: This is the most crucial cytokine in eosinophilic asthma. Its primary role is to act as a growth factor for eosinophils. It stimulates their production in the bone marrow, helps them mature, and directs their migration to the airways. Once in the lungs, IL-5 also activates the eosinophils, causing them to release toxic proteins and pro-inflammatory mediators that cause significant damage to the airway lining and contribute to the overall inflammation.
The Eosinophilic Effect
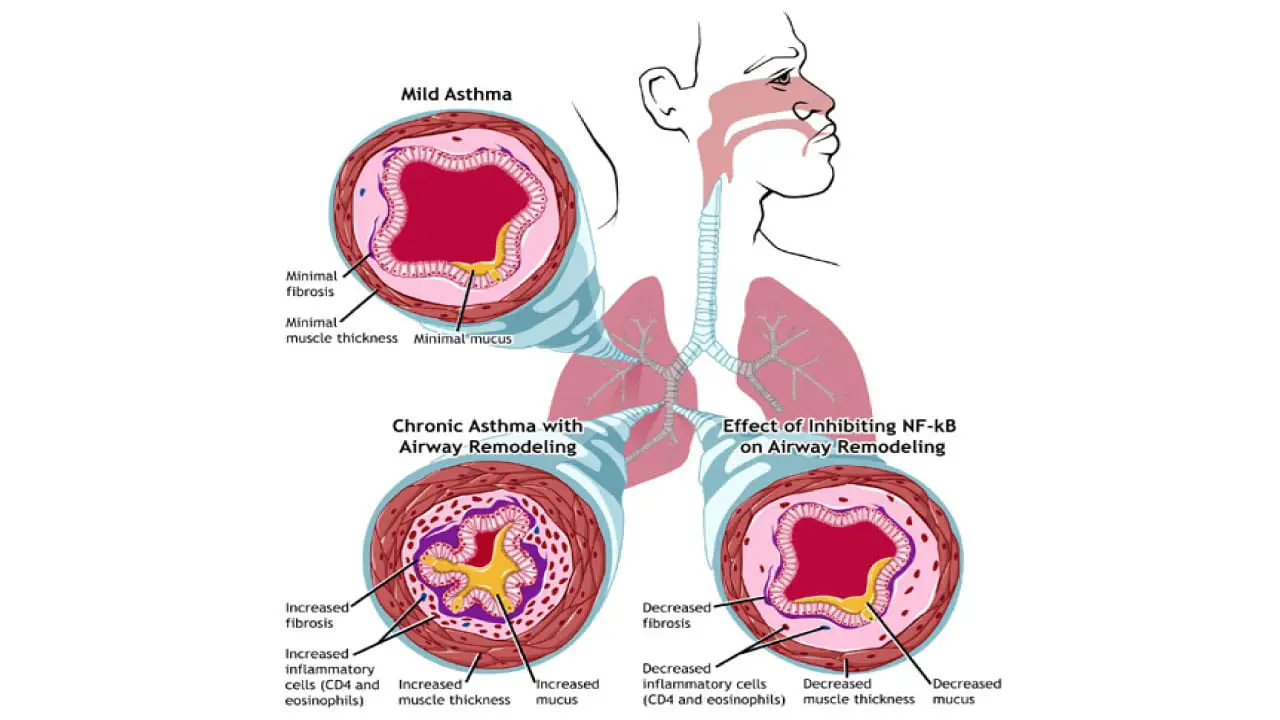
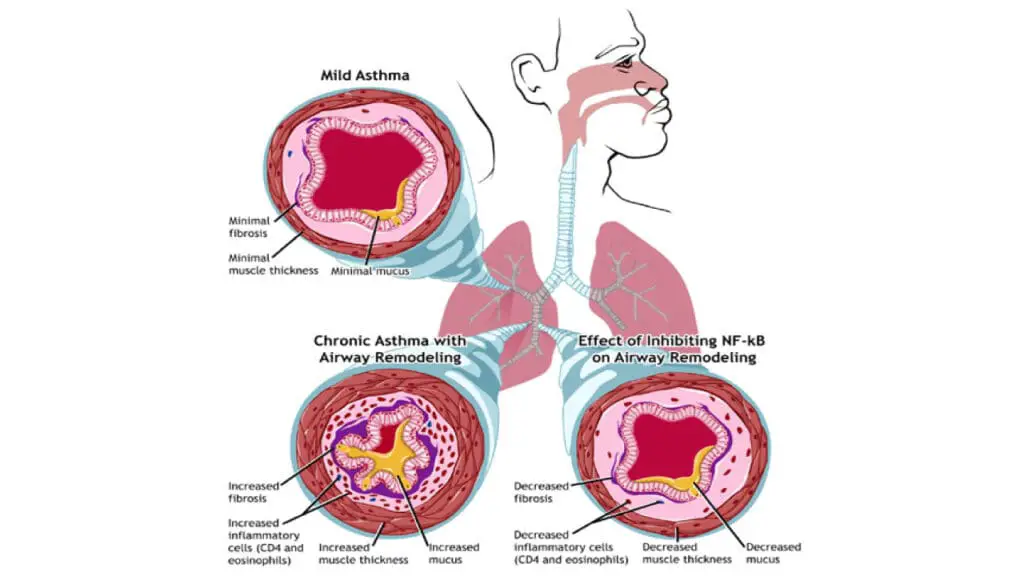
The presence of a large number of activated eosinophils in the airways is what defines this subtype of asthma. When these cells degranulate (release their contents), they cause tissue damage and contribute to the structural changes in the airways over time, a process known as airway remodeling. This is why eosinophilic asthma is often severe and difficult to manage with standard therapies alone. The entire process is a self-perpetuating cycle of inflammation and tissue damage, all driven by the T2 immune response and particularly the IL-5 pathway.
Causes and Risk Factors of Eosinophilic Asthma
The exact cause of eosinophilic asthma is still under investigation, but it’s understood to be a complex interplay between genetic predisposition and a variety of environmental factors.
Genetic Predisposition
Genetics play a significant role in a person’s susceptibility to asthma, with heritability rates in the adult population of up to 60%. While there isn’t a single “eosinophilic asthma gene,” a person’s genetic makeup can influence their immune system’s response.
For example, specific variations in genes that regulate the production of certain cytokines, such as Interleukin-4 (IL-4) and Interleukin-13 (IL-13), may increase the likelihood of developing T2 inflammation and, consequently, eosinophilic asthma. A family history of asthma, allergic rhinitis (hay fever), or atopic dermatitis (eczema) also increases the risk.
Allergic vs. Non-Allergic Triggers
- Allergic Eosinophilic Asthma: This type is linked to common allergens, such as pollen, dust mites, pet dander, and mold. In these cases, the body’s immune system overreacts to the allergen, triggering the T2 inflammatory cascade and leading to a high eosinophil count.
- Non-Allergic Eosinophilic Asthma: This is a key feature of this subtype, as it often presents without a clear allergic trigger. In these instances, the asthma is not caused by an allergic reaction but rather by other factors that still activate the T2 inflammatory pathway. This form often has a later onset, typically in adulthood, and is less responsive to standard allergy-focused treatments. The high eosinophil count is driven by the internal inflammatory process, not by an external allergen.
Environmental Factors
Environmental factors can both trigger and aggravate eosinophilic asthma. These are often non-allergic in nature but can still lead to the inflammatory response characteristic of the disease.
- Respiratory Infections: Viruses, particularly rhinoviruses, can trigger and worsen the inflammatory response, leading to an increase in eosinophils.
- Air Pollution: Exposure to irritants like diesel exhaust particles and other air pollutants can induce oxidative stress and chronic inflammation in the airways, contributing to the development or worsening of eosinophilic asthma.
- Tobacco Smoke: Both personal smoking and exposure to secondhand smoke are well-established risk factors for asthma development and severity.
- Occupational Exposure: Certain occupations that involve exposure to chemicals, dust, or fumes can be a risk factor for adult-onset asthma, including the eosinophilic subtype.
Signs and Symptoms of Eosinophilic Asthma
Eosinophilic asthma often presents with a distinct set of clinical features that differentiate it from other asthma subtypes. Because of the persistent inflammation driven by eosinophils, the symptoms are often more severe and less responsive to standard treatments.
Typical Features
The most common signs and symptoms mirror those of general asthma, but they tend to be more pronounced and refractory to treatment.
- Wheezing
- Shortness of Breath (Dyspnea)
- Chest Tightness
- Persistent Coughing
A key feature is that these symptoms are often late-onset, typically developing in adulthood rather than childhood.
Furthermore, patients with eosinophilic asthma frequently show a poor response to standard inhaled corticosteroids (ICS), which is a major red flag that points toward this specific phenotype.
Co-morbidities in Eosinophilic Asthma
Another important clinical characteristic is the frequent co-occurrence of other conditions related to T2 inflammation. These co-morbidities often provide additional clues for diagnosis
- Chronic Rhinosinusitis with Nasal Polyps: Inflammation of the nasal and sinus passages with the presence of benign growths. This is a very common co-morbidity in eosinophilic asthma.
- Atopic Dermatitis (Eczema)
- Allergic Rhinitis (Hay Fever)
The presence of these conditions, along with severe asthma symptoms and a poor response to inhaled corticosteroids (ICS), should prompt investigation into a potential diagnosis of eosinophilic asthma.
Laboratory and Diagnostic Investigations
Diagnosing eosinophilic asthma goes beyond clinical symptoms and involves a series of tests to confirm the presence of high eosinophil levels and the underlying type 2 inflammation.
Blood Eosinophil Count through Complete Blood Count
This is the most common and accessible test. It’s part of a standard complete blood count (CBC) with a differential.
While an elevated blood eosinophil count (typically >300 cells/μL) suggests eosinophilic inflammation, it’s not a definitive diagnosis on its own. It’s particularly useful as a biomarker to identify patients who are likely to respond to certain biologic therapies, such as anti-IL-5 agents.
Sputum Eosinophil Count
This test is considered the gold standard for diagnosing eosinophilic asthma. It involves collecting a sputum sample, often after inducing a cough, and analyzing it under a microscope to count the percentage of eosinophils.
A result of >3% eosinophils in the sputum is highly indicative of eosinophilic asthma. While highly accurate, this test is more complex, time-consuming, and requires specialized lab equipment and expertise.
Fractional Exhaled Nitric Oxide (FeNO)
The FeNO test is a simple, non-invasive breath test that measures the amount of nitric oxide (NO) in exhaled breath.
Elevated FeNO levels reflect inflammation in the airways that is driven by the cytokine IL-13. A high FeNO reading, typically >25 ppb in adults, can be a strong indicator of eosinophilic airway inflammation and is often used in conjunction with blood tests to support a diagnosis.
Pulmonary Function Tests (PFTs)
Pulmonary function tests, such as spirometry, measure lung function and airflow. While not specific to eosinophilic asthma, PFTs show the degree of airway obstruction and can reveal a poor or minimal response to bronchodilator medication.
In eosinophilic asthma, this often presents as more severe and less reversible airway obstruction, which is a key sign of the underlying, persistent inflammation.
Immunoglobulin E (IgE) Levels
Total serum IgE levels are measured via a blood test. While high IgE is characteristic of allergic (atopic) asthma, it is not a direct marker for eosinophilic asthma. However, it can provide additional information, as many cases of eosinophilic asthma have an underlying allergic component.
This test is most relevant for identifying patients who may be candidates for anti-IgE biologic therapies.
Differential Diagnosis of Eosinophilic Asthma
Non-Eosinophilic Asthma
Asthma is a broad category, and eosinophilic asthma is just one phenotype. Other types include neutrophilic asthma, which is characterized by a high number of neutrophils in the airways, and paucigranulocytic asthma, where there is little to no inflammatory cell infiltration. These subtypes generally do not respond well to corticosteroids and have different underlying inflammatory pathways.
Allergic Bronchopulmonary Aspergillosis (ABPA)
This is an immune-mediated disorder caused by a hypersensitivity reaction to the fungus Aspergillus fumigatus. It is most common in people with pre-existing asthma or cystic fibrosis. Like eosinophilic asthma, it presents with high eosinophil counts, but it is also characterized by recurrent pulmonary infiltrates, elevated serum IgE levels, and specific IgE antibodies against Aspergillus.
Eosinophilic Granulomatosis with Polyangiitis (EGPA)
Formerly known as Churg-Strauss Syndrome, EGPA is a rare form of vasculitis that affects small and medium-sized blood vessels. It typically has three phases: a prodromal phase with allergic rhinitis and asthma, an eosinophilic phase with high blood eosinophil counts and tissue infiltration, and a vasculitic phase affecting various organs. The presence of anti-neutrophil cytoplasmic antibodies (ANCA) can help distinguish EGPA from pure eosinophilic asthma.
Other Causes of Eosinophilia
- Drug reactions: Certain medications can trigger a temporary increase in eosinophils.
- Parasitic infections: Various parasitic diseases can cause significant eosinophilia.
- Hypereosinophilic syndromes: These are a group of rare disorders characterized by persistently high eosinophil counts for an extended period, which can lead to multi-organ damage.
Treatment and Management of Eosinophilic Asthma
Treatment for eosinophilic asthma is a step-wise approach that often requires a combination of therapies, moving from standard asthma medications to more targeted, specialized treatments. The primary goal is to control the eosinophilic inflammation, reduce symptoms, and prevent severe exacerbations.
Standard Asthma Therapies
The initial treatment for all asthma, including eosinophilic asthma, typically involves inhaled corticosteroids (ICS). These medications work to reduce general airway inflammation.
For many patients, a combination of an ICS and a long-acting beta-agonist (LABA) is used for daily symptom control. However, a hallmark of severe eosinophilic asthma is that it is often poorly controlled by these standard therapies alone.
In cases of severe exacerbations, oral corticosteroids (OCS) may be prescribed. While effective at reducing inflammation, long-term use of OCS is associated with significant side effects. Therefore, one of the main goals of modern management is to be oral corticosteroid-sparing.
Targeted Biologic Therapies
For patients whose eosinophilic asthma is not well-controlled with standard medications, biologic therapies are a crucial next step.
These drugs are monoclonal antibodies that specifically target the inflammatory pathways driven by T2 cytokines, thus providing a personalized approach to treatment.
- Anti-IL-5 Agents: Interleukin-5 (IL-5) is a key cytokine responsible for the maturation, recruitment, and activation of eosinophils. Medications such as mepolizumab, reslizumab, and benralizumab work by either binding directly to IL-5 or to its receptor on the eosinophil surface. This effectively reduces the number of eosinophils in the blood and lungs, leading to a reduction in asthma exacerbations and improved lung function.
- Anti-IL-4/IL-13 Agents: Dupilumab is a biologic that targets the shared receptor for Interleukin-4 (IL-4) and Interleukin-13 (IL-13). These cytokines are central to the type 2 inflammatory response that drives eosinophilic asthma. By blocking this receptor, dupilumab inhibits multiple inflammatory pathways, making it effective for patients with both eosinophilic and allergic asthma phenotypes.
- Anti-TSLP Agents: Tezepelumab is a newer biologic that targets Thymic Stromal Lymphopoietin (TSLP), an upstream cytokine released by airway epithelial cells in response to triggers. TSLP initiates the entire T2 inflammatory cascade, so blocking it at this early stage can be effective across a broad range of asthma phenotypes, including eosinophilic asthma.
The choice of biologic therapy is tailored to the individual patient, considering factors like their blood eosinophil count, FeNO levels, and the presence of co-morbidities like nasal polyps.
Prognosis and Complications
Prognosis
For many years, severe eosinophilic asthma was associated with a poor prognosis due to its resistance to standard therapies.
Patients often experienced frequent, severe exacerbations, leading to hospitalizations and a significant decline in lung function over time. However, the development of biologic therapies has dramatically changed this.
With effective management, patients can achieve better symptom control, leading to a much-improved quality of life. They experience fewer asthma attacks, better sleep, and can participate in physical activities they may have previously avoided.
Biologic therapies, in particular, are very effective at reducing the frequency of severe exacerbations, which are a major source of morbidity. By controlling chronic eosinophilic inflammation, these treatments can help prevent the irreversible airway remodeling that leads to a progressive decline in lung function.
Complications
Left untreated or poorly managed, eosinophilic asthma can lead to several serious complications.
- Airway Remodeling: Chronic, uncontrolled inflammation and damage caused by eosinophils can lead to a permanent thickening and scarring of the airways. This process, known as airway remodeling, results in a fixed and irreversible obstruction, making breathing difficult even with medication.
- Frequent Exacerbations: The persistent inflammation makes the lungs highly sensitive to triggers, leading to frequent and severe asthma attacks. These exacerbations can be life-threatening and require emergency medical care.
- Oral Corticosteroid Dependence: To manage severe symptoms and exacerbations, patients may become dependent on oral corticosteroids. Long-term use of these drugs can lead to significant side effects, including osteoporosis, weight gain, diabetes, and cataracts.
- Psychosocial Impact: The chronic nature of the disease, frequent symptoms, and fear of exacerbations can have a profound impact on a person’s mental health, leading to anxiety, depression, and social isolation.
Frequently Asked Questions
What is the difference between asthma and eosinophilic asthma?
Eosinophilic asthma is a specific subtype of asthma, so all cases of eosinophilic asthma are also cases of asthma. The key difference lies in the underlying cause of the airway inflammation. In all forms of asthma, inflammation causes the airways to swell and narrow, leading to symptoms like coughing, wheezing, and shortness of breath.
In eosinophilic asthma, this inflammation is specifically driven by a high number of white blood cells called eosinophils. These cells are a natural part of the immune system, but in this condition, they are overactive and release inflammatory chemicals in the lungs and airways. Eosinophilic asthma is often severe, can be non-allergic, and may not respond well to standard asthma treatments like inhaled corticosteroids.
In contrast, “general” or non-eosinophilic asthma can be caused by a variety of triggers, including allergens (like pollen or pet dander), viruses, exercise, and environmental irritants. While eosinophils may still be present, the inflammation isn’t primarily caused by an overabundance of these specific cells.
| Feature | General Asthma (Non-Eosinophilic) | Eosinophilic Asthma |
| Primary Cause | Various triggers, including allergens, viruses, exercise, and irritants. | Excessively high levels of eosinophils in the blood, lungs, and mucus. |
| Inflammation | May involve a mix of different immune cells and inflammatory pathways. | Primarily driven by eosinophil-related inflammation. |
| Response to Treatment | Often responds well to standard inhaled corticosteroids and other common asthma medications. | May be resistant to standard treatments and often requires specialized biologic therapies that target eosinophils. |
| Common Triggers | Allergens (pollen, dust mites), cold air, exercise, respiratory infections. | Often occurs without a clear external trigger like an allergy. |
Can stress trigger eosinophilic asthma?
Yes, stress can absolutely trigger eosinophilic asthma, but it’s not a direct trigger in the same way as an allergen. Instead, stress acts as an amplifier, making the body’s inflammatory response to other triggers much worse. It does this by affecting the immune system and the neuroendocrine system.
What to avoid with eosinophilic asthma?
For managing eosinophilic asthma, it is important to identify and avoid triggers that can worsen symptoms and lead to flare-ups. The things to avoid can be categorized into environmental factors, certain lifestyle habits, and specific dietary and medication-related concerns.
Environmental Triggers
Avoid or minimize exposure to common allergens and irritants, which can cause inflammation in the airways.
- Allergens: This includes pollen, dust mites, mold spores, and pet dander. Use air purifiers, keep windows closed during high-pollen seasons, and clean regularly to reduce dust mites.
- Irritants: Stay away from tobacco smoke (including secondhand smoke), strong perfumes, chemical cleaners, and air pollution. These can irritate the sensitive airways of someone with eosinophilic asthma.
- Respiratory Infections: Viruses and bacteria, such as those that cause the common cold or flu, can trigger severe asthma exacerbations. Practicing good hygiene and getting an annual flu shot can help reduce this risk.
Lifestyle Factors
Certain lifestyle choices and conditions can also impact the severity of asthma.
- Stress: High levels of psychological stress can trigger immune responses that worsen asthma symptoms. Managing stress through relaxation techniques can be beneficial.
- Strenuous Exercise: While moderate activity is encouraged, intense exercise can sometimes trigger asthma symptoms.
- Obesity: Maintaining a healthy weight is important, as obesity can worsen asthma symptoms and make them harder to control.
Dietary Considerations
Some people with eosinophilic asthma may find that certain foods trigger their symptoms. This is often more related to food allergies than the asthma itself.
- Common Allergens: Foods like dairy, eggs, wheat, soy, nuts, and shellfish are common allergens that can sometimes trigger an immune response leading to asthma symptoms.
- Sulfites: These are preservatives found in foods and beverages like wine, dried fruits, and some pickled foods, and they can trigger asthma symptoms in some individuals.
Medications
While many asthma medications are beneficial, some over-the-counter drugs can be problematic for certain people with asthma.
- Aspirin and NSAIDs: A small percentage of people with asthma, particularly those with adult-onset eosinophilic asthma and nasal polyps, have a condition called Aspirin-Exacerbated Respiratory Disease (AERD). If the patient has this, the individual should avoid aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen.
Is eosinophilic asthma a disability?
The Americans with Disabilities Act (ADA) legally considers asthma, including eosinophilic asthma, a disability if it substantially limits one or more “major life activities” like breathing, working, or going to school. This protection is in place even if symptoms are intermittent and are managed with medication.
Who treats eosinophilic asthma?
Eosinophilic asthma is typically treated by specialists who focus on respiratory and allergic conditions. The main types of doctors who treat this condition are pulmonologists, allergists, and immunologists.
Can you outgrow eosinophilic asthma?
No, you can’t technically “outgrow” eosinophilic asthma, as it’s considered a lifelong condition. However, it is possible for the symptoms to go into remission, especially in children. The prognosis for eosinophilic asthma is generally good, especially with effective management.
Disclaimer: This article is intended for informational purposes only and is specifically targeted towards medical students. It is not intended to be a substitute for informed professional medical advice, diagnosis, or treatment. While the information presented here is derived from credible medical sources and is believed to be accurate and up-to-date, it is not guaranteed to be complete or error-free. See additional information.
References
- Porpodis, K., Tsiouprou, I., Apostolopoulos, A., Ntontsi, P., Fouka, E., Papakosta, D., Vliagoftis, H., & Domvri, K. (2022). Eosinophilic Asthma, Phenotypes-Endotypes and Current Biomarkers of Choice. Journal of Personalized Medicine, 12(7), 1093. https://doi.org/10.3390/jpm12071093
- Ramphul, M., Lo, D. K. H., & Gaillard, E. A. (2021). Precision Medicine for Paediatric Severe Asthma: Current Status and Future Direction. Journal of asthma and allergy, 14, 525–538. https://doi.org/10.2147/JAA.S265657
- Oppenheimer, J., Hoyte, F. C. L., Phipatanakul, W., Silver, J., Howarth, P., & Lugogo, N. L. (2022). Allergic and eosinophilic asthma in the era of biomarkers and biologics: similarities, differences and misconceptions. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology, 129(2), 169–180. https://doi.org/10.1016/j.anai.2022.02.021
- Buhl, R., Humbert, M., Bjermer, L., Chanez, P., Heaney, L. G., Pavord, I., Quirce, S., Virchow, J. C., Holgate, S., & expert group of the European Consensus Meeting for Severe Eosinophilic Asthma (2017). Severe eosinophilic asthma: a roadmap to consensus. The European respiratory journal, 49(5), 1700634. https://doi.org/10.1183/13993003.00634-2017
- Coumou, H., & Bel, E. H. (2016). Improving the diagnosis of eosinophilic asthma. Expert review of respiratory medicine, 10(10), 1093–1103. https://doi.org/10.1080/17476348.2017.1236688
- Nopsopon, T., Lassiter, G., Chen, M. L., Alexander, G. C., Keet, C., Hong, H., & Akenroye, A. (2023). Comparative efficacy of tezepelumab to mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: A Bayesian network meta-analysis. The Journal of allergy and clinical immunology, 151(3), 747–755. https://doi.org/10.1016/j.jaci.2022.11.021
- Pavord, I., Gardiner, F., Heaney, L. G., Domingo, C., Price, R. G., Pullan, A., Oppenheimer, J., Brusselle, G., Nagase, H., Chupp, G., Pizzichini, E., Bañas-Conejero, D., & Howarth, P. (2023). Remission outcomes in severe eosinophilic asthma with mepolizumab therapy: Analysis of the REDES study. Frontiers in immunology, 14, 1150162. https://doi.org/10.3389/fimmu.2023.1150162
- Yancey, S. W., Keene, O. N., Albers, F. C., Ortega, H., Bates, S., Bleecker, E. R., & Pavord, I. (2017). Biomarkers for severe eosinophilic asthma. The Journal of allergy and clinical immunology, 140(6), 1509–1518. https://doi.org/10.1016/j.jaci.2017.10.005