Introduction
When a blood vessel is injured, platelets rush to the scene, forming a sticky plug to seal the wound and prevent blood loss. This vital process is called platelet aggregation, and its effectiveness is often assessed through a crucial test: the platelet aggregation test (PAT).
The PAT acts as a diagnostic tool to evaluate the platelet’s ability for proper clot formation. A dysfunction in aggregation can lead to excessive bleeding or even the formation of unwanted clots, both posing significant health risks.
Why is the PAT Important?
The PAT provides valuable insights into various bleeding disorders like:
- Thrombocytopenia: A low platelet count, leading to excessive bleeding.
- Von Willebrand disease: A genetic disorder impacting the protein that helps platelets adhere to each other and the blood vessel wall.
- Bernard-Soulier syndrome: Another genetic disorder affecting the platelet surface proteins needed for aggregation.
- Antiplatelet medication: Assessing the effectiveness of medications used to prevent blood clots.
When Might the PAT be Indicated?
The PAT is often ordered when someone experiences:
- Unexplained or excessive bruising or bleeding.
- Prolonged bleeding after an injury or surgery.
- Recurrent miscarriages.
- A personal or family history of bleeding disorders.
The PAT, while a valuable tool, is often used in conjunction with other tests and a comprehensive clinical evaluation to form a conclusive diagnosis.
Principle of Platelet Aggregation Test (PAT)
The test hinges on the use of platelet-rich plasma (PRP), a concentrated suspension of platelets extracted from the patient’s blood. This PRP is then exposed to various agonists, substances that trigger platelet activation and aggregation. These agonists mimic the natural signals that platelets encounter at the site of an injury, such as collagen exposed from a damaged blood vessel.
As the agonists enter the PRP, they bind to specific receptors on the platelet surface. This binding triggers a cascade of events within the platelet, leading to a dramatic transformation. The platelet’s internal machinery kicks into gear, causing it to change shape, sprout spiky protrusions called pseudopodia, and release adhesive molecules to get other platelets to aggregate together. This aggregation is monitored by the aggregometer.
By measuring the changes in the electrical impedance of the PRP, the test tracks the formation and growth of platelet clumps. The speed and extent of aggregation, captured in a graph called an aggregogram, provide crucial insights into the health and function of the platelets evaluated against a control.
Materials
- Platelet-rich plasma (PRP)
- Agonists: Adenosine disphosphate (ADP) (2.5 µmol/L), Collagen (1.25 µg/mL), Epinephrine (5.00 µmol/L), Arachidonic Acid (AA) (1.0 mmol/L), Ristocetin sulfate (1.2 mg/mL) (optional)
- Aggregation analyzer (aggregometer)
- Calibrator plasma (Control)
- Disposable pipettes
- Test tubes
- Timer
- Centrifuge
- Trisodium citrate vacutainer
- Water bath (37°C)
Protocol
Platelet-rich plasma (PRP) preparation
To ensure accurate results for the platelet aggregation test, patient and control subjects should avoid food, beverages, and medications that could affect platelet function for 10 days before the test and fast overnight prior to sample collection.
The test window for platelet aggregation is 30 minutes after PRP preparation and must be completed within 4 hours of blood collection.
- Collect 20 mL whole blood in an trisodium citrate-anticoagulated tube. Blood to trisodium citrate ratio should be 9:1.
- Centrifuge the blood at 1500 – 2000 g (3000 rpm) for 15 minutes at room temperature.
- Carefully aspirate the PRP layer without disturbing the buffy coat or red blood cells.
- Pipette the PRP into a test tube and warm it to 37°C in a water bath.
- Centrifuge the remaining blood at 2000 – 2500 g for 20 minutes at room temperature for platelet poor plasma (PPP).
- Carefully aspirate the PPP layer without disturbing the buffy coat or red blood cells.
- Pipette the PPP into a test tube and warm it to 37°C in a water bath.
- Check the platelet count for PRP and carefully dilute the PRP with PPP until the platelet count is approximately 200 – 400 x 109/L.
Platelet aggregation
- Pre-heat the aggregometer block to 37°C for 30 minutes prior to starting the test.
- Place a magnetic stir bar into each glass cuvette.
- To establish a baseline of 0% and 100%, pipette 500 μL of PPP and PRP, respectively, into separate cuvettes. Measure and then remove them.
- Pipette 450 μL of patient and control PRP into their designated channels (Channel 1: Control, Channel 2: Patient). For each agonist test, prepare a paired set of cuvettes.
- Set the stirring speed to 900 rpm and incubate at 37°C for 3 minutes.
- Monitor the aggregometer baseline and adjust as needed.
- Add the agonist (e.g., ADP) and record the start time for both test and control PRP. Run the aggregometer for a maximum of 8 minutes before stopping the test.
- Repeat the procedure with each agonist at different concentrations, following the manufacturer’s instructions.
- Record the results for each agonist and compare them to the reference values.
Optional tests
- Perform additional aggregation tests with specific inhibitors to identify the cause of abnormal aggregation.
Interpretation
Normal Platelet Aggregation Response:
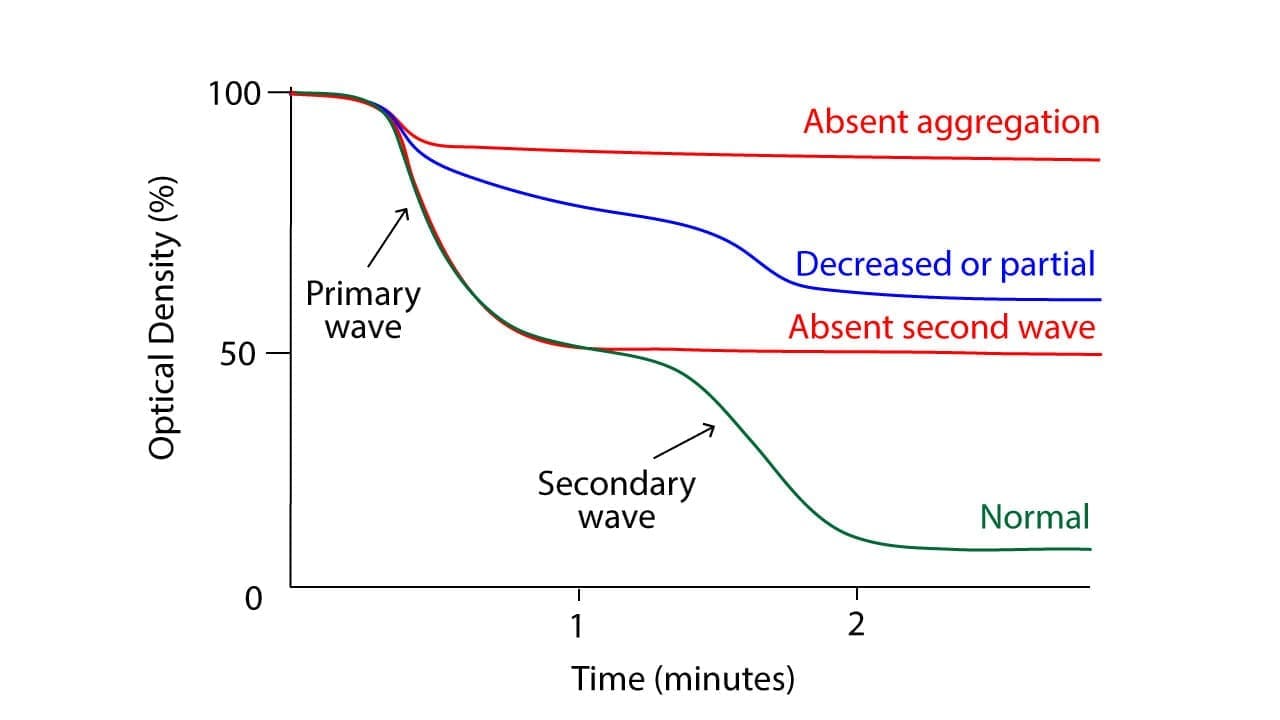
The normal platelet aggregation response varies slightly depending on the agonist used:
- ADP: Triggers a biphasic response. The first phase is a quick, small aggregation due to ADP-mediated activation of receptors. The second phase, larger and delayed, is caused by released thromboxane A2 and further platelet activation.
- Collagen: Induces a rapid, sustained single phase aggregation due to direct activation of collagen receptors on platelets.
- Ristocetin: Mediates a specific clumping through von Willebrand factor (vWF), resulting in a moderate and rapid aggregation.
- Arachidonic Acid: Induces a slow aggregation via the thromboxane A2 pathway, similar to the second phase of the ADP response.
- Epinephrine: Produces a weak and variable aggregation through alpha-adrenergic receptors, primarily seen in high concentrations.
Abnormalities in the aggregogram (the graph depicting platelet aggregation over time) can indicate various platelet function disorders:
- Hypersensitivity: Increased amplitude or faster onset of aggregation suggests platelet hyperactivity or excessive sensitivity to the agonist.
- Hyposensitivity: Weak or absent aggregation implies impaired platelet function or response to the agonist.
- Delayed aggregation: Can indicate abnormalities in specific activation pathways within the platelets.
- Absence of second phase: Suggests defects in thromboxane A2 production or signaling.
Technical Artefacts
Several factors can influence the test results and be misinterpreted as abnormalities:
- Improper platelet-rich plasma (PRP) preparation: Can lead to platelet activation or decreased concentration, affecting aggregation.
- Temperature variations: Outside the optimal range (usually 37°C) can alter platelet activity.
- Stirring speed: Incorrect stirring can affect platelet interaction and aggregation dynamics.
- Agonist concentration: Improper concentration can lead to over- or under-stimulation of platelets.
- Instrument calibration: Errors in calibration can skew the measured aggregation values.
Therefore, interpreting platelet aggregation test results requires careful consideration of the entire aggregogram, clinical context, and potential technical artefacts. Evaluating the response to different agonists further aids in differentiating specific platelet function disorders.
Note: This is a general overview, and specific interpretation of results depends on the laboratory’s reference values and established practices. Always consult a qualified healthcare professional for diagnosis based on individual results.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- Koltai K, Kesmarky G, Feher G, Tibold A, Toth K. Platelet Aggregometry Testing: Molecular Mechanisms, Techniques and Clinical Implications. Int J Mol Sci. 2017 Aug 18;18(8):1803. doi: 10.3390/ijms18081803. PMID: 28820484; PMCID: PMC5578190.
- https://practical-haemostasis.com/Platelets/platelet_function_testing_lta.html
- Saba HI, Roberts HR. Hemostasis and Thrombosis: Practical Guidelines in Clinical Management (Wiley Blackwell). 2014.