Procedure-at-a-Glance
| Step | Action |
| Preparation | Mix equal parts blood and stain (BCB or NMB) |
| Incubation | 15 – 30 minutes |
| Re-suspension | Mix the tube |
| Smear | Create a thin film |
| Drying | Air dry |
| Examination | Oil Immersion (100x) |
Introduction
Brilliant Cresyl Blue also known as BCB and new methylene blue (NMB) stains are also known as supravital stains. These stains are commonly used to stain reticulocytes, Heinz bodies or H inclusions. Enumeration of reticulocytes is a simple technique to assess bone marrow activity in producing red blood cells. Higher reticulocyte count compared to normal infers a high erythropoietic activity in the bone marrow. Commonly, reticulocytosis occurs following acute blood loss or hemolysis, in chronic anemias or also in response to treatment with recombinant erythropoietin.
In a modern diagnostic lab, NMB is the preferred choice for manual reticulocyte counts. Because NMB is more chemically consistent, it reduces “inter-observer variation” which means two different technicians are more likely to get the same result when looking at the same slide. The filaments it creates are much darker and more distinct against the pale background of the RBC, making it easier to distinguish a true reticulocyte from a cell with a small artifact or “junk” on the slide.
While NMB wins for reticulocytes, BCB is the hero of Thalassemia screening. When a lab is looking for Hemoglobin H (HbH) inclusions, BCB is the superior reagent. BCB excels at inducing the precipitation of unstable Hemoglobin H. Under a BCB stain, these cells look like “pitted golf balls” or “raspberries.” While NMB can show these, BCB is traditionally more sensitive for this specific diagnostic task.
BCB vs. NMB
| Feature | Brilliant Cresyl Blue (BCB) | New Methylene Blue (NMB) |
| Chemical Nature | An oxazine dye; often contains impurities and varying concentrations. | A thiazine dye; highly purified and chemically stable. |
| CLSI Status | Not designated as the reference standard for manual counts. | Designated Reference Method (CLSI H44-A2) for manual reticulocyte counting. |
| Reticulum Appearance | Stains filaments/granules a mid-to-light blue; can be faint. | Stains reticulum a dark blue or blue-black; much higher contrast. |
| Background (RBC) | Erythrocytes typically appear pale blue-green. | Erythrocytes typically appear pale greenish-yellow. |
| Main Clinical Strength | Gold Standard for HbH Inclusions. Highly sensitive for detecting “golf ball” cells in Alpha-Thalassemia. | Gold Standard for Reticulocytes. Preferred for routine monitoring of bone marrow activity. |
| Oxidative Properties | Acts as a stronger oxidant, which helps in the precipitation of unstable HbH. | A milder oxidant; better for general ribosomal RNA precipitation. |
| Precision | Slightly lower; inter-observer variation is higher due to lighter staining. | Higher precision; sharp outlines make it easier for different techs to count consistently. |
| Precipitation | Prone to forming dye crystals; must be filtered frequently. | More soluble, though filtering is still recommended to avoid artifacts. |
| Heinz Body Detection | Excellent; highlights them as single, dark dots. | Excellent; often preferred because they stand out more against the background. |
Principle of Supravital Stain
The principle of supravital staining relies on the ability of specific basic dyes, such as Brilliant Cresyl Blue (BCB) or New Methylene Blue, to penetrate the membranes of living (unfixed) cells and interact with intracellular components. Unlike standard Romanowsky stains (like Wright-Giemsa) that require cells to be killed and “fixed” with alcohol, supravital dyes (BCB or NMB) are mixed with fresh, whole blood while the cells are still metabolically active.
The cationic dye molecules bind to acidic, anionic structures most notably the ribosomal RNA remnants in immature red blood cells. This interaction causes the RNA to precipitate and clump together into a visible, dark blue network of granules and filaments called a reticulum. This allows the laboratory technician to visualize and quantify reticulocytes or identify specific inclusions like Heinz bodies and HbH “golf ball” bodies that would remain invisible on a routine fixed smear.
Method differs slightly according to the manufacturer’s protocol.
Materials
- 1.0% New Methylene Blue (NMB) OR 1.0% Brilliant Cresyl Blue (BCB) (filtered).
- 1 mL of EDTA peripheral blood
- 1 glass test tube (10 m x 75 mm)
- Pasteur pipette
- Dry incubator set at 37oC
- Hair dryer
- A clean glass slide
Protocol
Standard Supravital Staining Protocol
Use this for routine reticulocyte counting with either New Methylene Blue (NMB) or Brilliant Cresyl Blue (BCB).
- Always filter a small amount of the dye before use to remove any precipitated crystals that could be mistaken for inclusions.
- Place 2 to 3 drops of the filtered stain into a small test tube. Add an equal volume (2 to 3 drops) of well-mixed EDTA blood.
- Gently flick the bottom of the tube to mix the blood and stain thoroughly.
- Incubate. For NMB: Let the mixture sit at room temperature for 10–15 minutes. For BCB: Let the mixture sit at room temperature for 15–30 minutes.
- After incubation, resuspend the contents of the tube using a Pasteur pipette and use 1 drop of the mixture to make a thin smear on a clean glass slide.
- Dry the slide using the hair dryer on the lowest speed or air dry in a tilted position.
- This slide is now ready for viewing using oil immersion under a high power (X100) lens.
*After 15 minutes of incubation time, reticulin and Heinz bodies will be visible. Heinz bodies will stain dark blue-purple. However, further incubation time of BCB stain for approximately 2 hours is required if H inclusions are required for confirmation. H inclusions will appear like dark blue golf balls within the red blood cells. Please note that the H inclusion test cannot be performed on a stored sample.
Specialized Protocol: BCB for HbH Inclusions
This protocol is used specifically to screen for Alpha-Thalassemia (Hemoglobin H disease).
- Follow the 1:1 ratio of BCB stain and EDTA blood in a test tube.
- Place the tube in a 37°C incubator.
- Prepare smears at multiple intervals: 1 hour, 2 hours, and 4 hours. *Some HbH inclusions precipitate quickly, while others require longer “oxidative stress” to appear.
- Dry the slide using the hair dryer on the lowest speed or air dry in a tilted position.
- This slide is now ready for viewing using oil immersion under a high power (X100) lens. Look for the characteristic “Golf Ball” appearance (multiple small, greenish-blue dots distributed throughout the RBC).
Interpretation
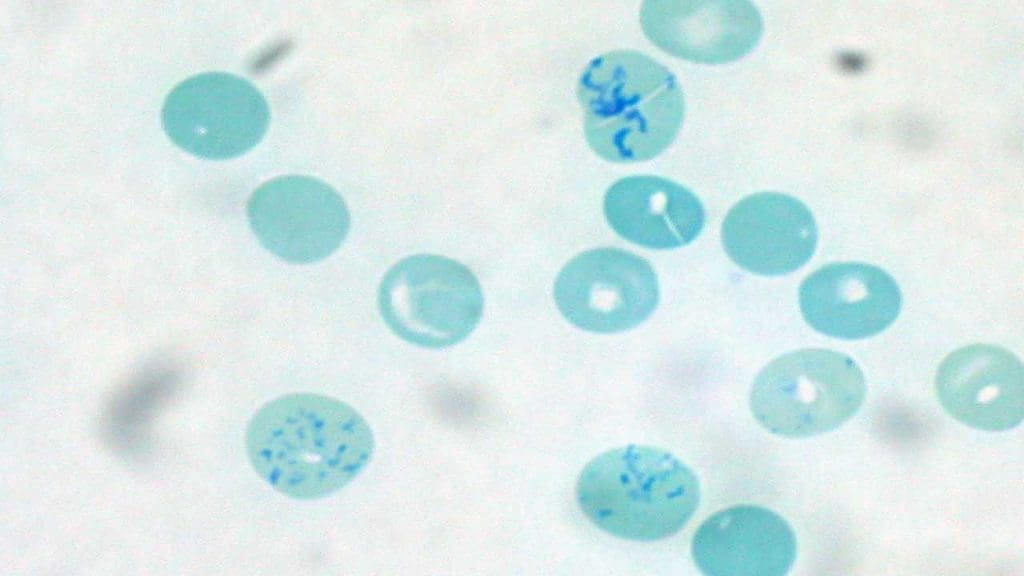
The normal reference range for reticulocytes count in a normal adult is 35 – 108 x 109/L (0.5 – 2.1%). In infants whether full term or using cord blood the range is 2 – 5%.
A common mistake in the lab is relying solely on the Reticulocyte Percentage. Because this is a relative number (the ratio of reticulocytes to mature RBCs), it can be deceiving. If a patient has severe anemia (fewer mature RBCs), their reticulocyte percentage will look high even if the bone marrow isn’t producing any new cells. This is known as the “anemia trap.” To get the true story, we must use ARC and RPI.
Absolute Reticulocyte Count (ARC)
The ARC represents the actual, physical number of reticulocytes present in a specific volume of blood (usually per microliter). By looking at the ARC instead of the percentage, we remove the influence of the total red cell count. It tells you exactly how many “new recruits” are entering the circulation right now.
Formula
ARC = (Reticulocyte %/100) x RBC Count
- Example: If a patient has a 2.0% reticulocyte count and an RBC count of 4.0 x 10⁶/µL:
- 0.02 x 4,000,000 = 80,000/µL
- Normal Range: Approximately 20,000–100,000/µL (or 25 – 75 x 109/L).
Reticulocyte Production Index (RPI)
While the ARC is a great first step, the RPI is the gold standard for clinical decision-making. It goes one step further by correcting for “Shift Reticulocytes.” When a patient is severely anemic, the bone marrow gets “desperate” and releases reticulocytes into the blood prematurely. These young cells stay in the blood for 2 to 3 days before maturing, instead of the usual 24 hours. Without the RPI correction, we would count these cells multiple days in a row, leading to a falsely optimistic view of the bone marrow’s performance. The RPI “normalizes” the data to tell us how many reticulocytes the marrow is producing per day.
Formula
RPI = Corrected Retic %/Maturation Factor
Corrected Reticulocyte Count (CRC)
In anemia, the percentage of reticulocytes may look high simply because there are fewer mature RBCs to compare them to. This formula adjusts the percentage based on a “normal” hematocrit (Hct), typically 45%.
Formula
Corrected Retic % = Observed Retic % x (Patient’s Hct/Normal Hct (45))
Maturation Correction Table
Use the patient’s hematocrit to determine the correct Maturation Factor (Shift Correction)
| Patient’s Hematocrit (Hct) | Maturation Factor (Days) |
| 45% | 1.0 |
| 35% | 1.5 |
| 25% | 2.0 |
| 15% | 2.5 |
Clinical Correlation: Reticulocyte Results & Disease States
| Bone Marrow Response | RPI / ARC Value | Clinical Meaning | Associated Conditions |
| Regenerative | RPI > 3.0 | The marrow is healthy and producing RBCs at an accelerated rate to compensate for loss. | • Hemolytic Anemia (G6PD, Sickle Cell, AIHA) • Acute Blood Loss (Hemorrhage) • Post-Treatment (Recent Iron or B12 therapy) |
| Inadequate | RPI 2.0 – 3.0 | The marrow is attempting to respond but is not keeping up with the severity of the anemia. | • Mild Blood Loss • Early recovery from bone marrow suppression. |
| Non-Regenerative | RPI < 2.0 | The marrow is either damaged or lacks the “building blocks” (iron/vitamins) to produce cells. | • Iron Deficiency Anemia • Aplastic Anemia • Megaloblastic Anemia (B12/Folate deficiency) • MDS (Myelodysplastic Syndromes) |
Troubleshooting: Common Manual Staining Errors
| The Observation | The Likely Cause | The Solution |
| Faint or non-existent reticulum | Under-incubation or too much blood in the ratio. | Ensure 15–30 mins for BCB. Check blood-to-stain ratio; ensure cells aren’t “drowned” by too many RBCs. |
| Highly refractive “shiny” spots | Moisture in the slide or humidity during drying. | Air-dry slides quickly. In humid climates, use a small fan or hair dryer (cool setting) to speed up drying. |
| Dark blue “chunks” on the slide | Dye precipitate (common with BCB). | Always filter the stain immediately before adding it to the blood. |
| Cells are overlapping/stacked | Smear is too thick (using packed cells or too large a drop). | Use whole blood, not packed cells. Ensure you are using a small drop and viewing the monolayer. Please read our article on Preparation of Peripheral Blood Smears |
| Inclusions at the cell edge only | These are likely Heinz Bodies, not reticulum. | Remember: Heinz bodies are large and marginal; reticulum is a central network or “mesh.” Please read our article on Red Blood Cell Inclusion Bodies for further information. |
| Small, irregular dark purple dots | Likely Pappenheimer Bodies (siderotic granules). | Check a Wright-Giemsa stain; Pappenheimer bodies stay visible on Wright stain, whereas reticulum does not. |
Frequently Asked Questions (FAQs)
Why can’t I see reticulocytes on a standard Wright-Giemsa stain?
Wright-Giemsa stain fixes the cells first. You can see “polychromasia” (blue-gray cells), but the dye cannot clump the RNA into the visible “net-like” reticulum. Only supravital stains like BCB or NMB can precipitate the RNA in living cells to make it visible.
What is the difference between Brilliant Cresyl Blue (BCB) and New Methylene Blue?
New Methylene Blue (NMB) is generally preferred for routine reticulocyte counting because it is more chemically pure and provides a darker, more distinct stain. Brilliant Cresyl Blue (BCB) is specifically valued for its ability to highlight Hemoglobin H inclusions.
Do I need to fix the slide with alcohol after staining?
No. Fixing the slide can interfere with the supravital dye’s appearance. The smear should be air-dried and viewed directly under oil immersion.
How does the patient’s hematocrit affect the staining procedure?
For patients with very low hematocrit (anemia), you should use a higher ratio of blood to stain (e.g., 4 drops of blood to 2 drops of stain) to ensure there are enough cells to count on the slide.
Can I use these stains to see Howell-Jolly bodies?
While they may be visible, Howell-Jolly bodies (DNA remnants) are best viewed on a standard Wright-stained smear. Supravital stains are specifically designed for RNA (reticulocytes) and denatured hemoglobin (Heinz bodies/HbH).
Glossary of Medical Terms
- Supravital Stain: A staining technique used on living cells that have been removed from the body but are not yet fixed (killed).
- Reticulocyte: An immature, non-nucleated red blood cell that still contains remnants of ribosomal RNA.
- Polychromasia: The bluish-gray tint of young RBCs on a standard Wright-Giemsa stain, signifying increased erythropoiesis.
- Heinz Bodies: Denatured hemoglobin inclusions (usually caused by G6PD deficiency or oxidative stress) that appear as single, large dots on the edge of the cell when stained supravitally.
- HbH Inclusions: Multiple, small “golf ball-like” dots throughout the RBC, characteristic of Hemoglobin H disease (Alpha-Thalassemia).
- Erythropoiesis: The process of red blood cell production in the bone marrow.
- Fixation: The process of using chemicals (like methanol) to preserve cell structure. Note: Supravital staining must be done before fixation.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.




