Introduction
Antibody screening, a cornerstone of blood transfusion safety, aims to detect clinically significant unexpected red blood cell (RBC) antibodies in a patient’s plasma or serum. These antibodies can develop through various mechanisms, including prior blood transfusions, fetomaternal hemorrhage (in pregnancy), or autoimmune processes. When present, these antibodies can bind to antigens on transfused RBCs, leading to agglutination (clumping) or hemolysis (destruction) of the transfused cells. This can cause serious complications such as hemolytic transfusion reactions, reduced red blood cell survival, and even death.
Principle of Antibody Screening
The principle behind antibody screening relies on in vitro antigen-antibody interaction. The test exposes the patient’s plasma or serum to a panel of RBCs with known antigen profiles. These screening cells are typically type O red blood cells, chosen for their lack of A and B antigens, minimizing interference from the patient’s ABO blood group. The screening panel includes cells with various clinically significant antigens, such as the Rh system (D, C, E, c, e), Kell (K), Duffy (Fy), Kidd (Jk), MNS (M, N, S, s), Lewis (Lea, Leb), and P1.
The methodology of antibody screening involves three phases:
- Immediate Spin: This step detects antibodies, primarily IgM, which react best at room temperature. Patient plasma/serum is incubated with screening cell suspensions for a short period (around 15 seconds) followed by centrifugation. Agglutination observed after resuspension indicates a possible antibody.
- Incubation: The remaining tubes are incubated at 37°C for a longer duration (typically 15-30 minutes). This facilitates the detection of antibodies belonging to the IgG class, which have a slower reaction time but are more clinically relevant due to their ability to activate complement and cause hemolysis.
- Antiglobulin Phase (AHG): After incubation, unbound patient proteins are washed away. An antiglobulin reagent, containing antibodies specific to human IgG on red blood cells, is added. If patient IgG antibodies are bound to the screening cells, the antiglobulin reagent will crosslink them, leading to visible agglutination, even if it wasn’t apparent earlier.
Following these steps, the results are interpreted. Agglutination in any phase suggests the presence of an antibody. Further identification of the specific antibody is crucial for safe blood transfusion practices. This involves testing the patient’s plasma/serum against a wider panel of RBCs with defined antigen profiles to pinpoint the exact antibody specificity.
By implementing this protocol, we can effectively detect clinically significant unexpected red blood cell antibodies in patients. This information is vital for selecting compatible blood components for transfusion, minimizing the risk of adverse reactions, and ensuring patient safety.
Materials
- Patient plasma or serum. Fresh samples (ideally less than 3 days old) are preferred to ensure optimal reactivity of antibodies and complement proteins.
- Screening cell panel
- Test tubes or microplates (depending on the chosen method)
- Centrifuge
- Normal saline (0.9% NaCl)
- Anti-human globulin (AHG) solution
- Micropipettes and sterile tips
- Coombs control cells (CCC)
- Warm water bath or incubator for maintaining incubation temperature (37°C)
- Antigram result panel
- Low ionic strength solution (LISS) or polyethylene glycol (PEG) as potentiating agent (Optional as not universally used)
Reagent Criteria
This criteria is taken from BCSH Guideline: Pre-Transfusion Compatability Procedures (2012).
Cell Panel Composition
- A minimum of two different donor red blood cell sets are required for antibody screening.
- One set should be homozygous (R2R2(cDE/cDE)) for the Rh antigens (D, C, E). The other set can be either homozygous (R1R1 (CDe/CDe)) or weakly positive (R1wR1) for Rh antigens.
- Additionally, the screening cell panel must include cells that express the following antigens: K, k, Fya, Fyb, Jka, Jkb, S, s, M, N, P1, Lea, and Leb.
Individual Cell Use
- Pooled red blood cells from different donors should not be used for antibody screening. Each cell set should be tested individually.
Homozygous Antigen Expression
- To ensure high sensitivity for detecting certain antibodies, at least one cell within the panel must be homozygous for the Fya, Fyb, Jka, Jkb, S, and s antigens. This recommendation is based on data concerning delayed transfusion reactions and the need for sensitive detection of Kidd antibodies (reference: SHOT, 1996–2010; Knowles et al., 2002).
Storage and Preservation
- Red blood cells used for antibody screening must be stored in a temperature-controlled environment with a diluent proven to minimize the loss of blood group antigens throughout the recommended storage period.
Local Validation of Screening Cells
- Each laboratory should perform a local validation to ensure the stability of their chosen screening cells for routine use. This validation should apply to cells stored on analyzers, benches, or refrigerators.
Expiry Dating
- Based on the local validation results, a time limit for each opened bottle of screening cells should be established. This time limit should be reevaluated whenever storage conditions or usage patterns change.
Manufacturer’s Instructions
- All reagents, including screening cells, must be used within the expiry date specified by the manufacturer.
Protocol
This is a general outline of the antibody screening protocol. Specific details like incubation times and centrifugation speeds may vary depending on the laboratory and chosen methodology (tube or gel card technique). Always refer to the manufacturer’s instructions and established laboratory protocols for detailed procedures and interpretations.
Preparation
- Sample Collection and Processing: Collect a blood sample from the patient and centrifuge it to separate plasma or serum from red blood cells. Ideally, use fresh samples (less than 3 days old) for optimal antibody reactivity.
- Preparation of Reagents: Prepare the screening cell panel according to the manufacturer’s instructions. Dilute the red blood cell suspensions to the recommended concentration using saline solution. Prepare antiglobulin reagent (AHG) and other necessary reagents like saline and quality control materials.
Antibody Screening
Immediate Spin
- Label test tubes or microplates for each screening cell and a negative control (patient’s plasma/serum with saline).
- Add a drop of each diluted screening cell suspension to its designated tube/well.
- Add 2 drops of patient’s plasma/serum to all tubes/wells (excluding the negative control).
- Optional: Add 2 drops of LISS to all tubes/wells.
- Centrifuge the tubes/plate for a short duration (around 15 seconds depending on manufacturer’s instruction) at room temperature.
- Gently resuspend the cell pellet and visually inspect for agglutination. Record any observed agglutination.
- Incubate the remaining tubes/plate containing patient plasma/serum and screening cells at 37°C for a designated time (typically 15-30 minutes depending on manufacturer’s instruction). This allows detection of slower-reacting antibodies (IgG).
Antiglobulin Phase (AHG)
- After incubation, wash the tubes/plate with saline solution to remove unbound patient proteins. Discard the wash solution.
- Repeat the wash twice.
- Add a drop of AHG reagent to each tube/well (excluding the negative control).
- Centrifuge the tubes/plate again for a short duration (around 15 seconds depending on manufacturer’s instruction).
- Gently resuspend the cell pellet and visually inspect for agglutination. Record any observed agglutination.
- In the absence of agglutination after initial centrifugation, add Coombs control cells (CCC) and centrifuge as per manufacturer instructions. Observe and record any agglutination with CCC, confirming test validity.
Interpretation
- Agglutination in any phase (immediate spin, incubation, or AHG) suggests the presence of an antibody.
- A negative screen (no agglutination in any phase) indicates a low likelihood of clinically significant unexpected antibodies. However, further testing may be necessary depending on the clinical scenario.
| Result | Interpretation |
| Positive agglutination or hemolysis after incubation at 37°C or after AHG addition | Positive presence of an antibody |
| Negative agglutination after each phase but agglutination is observed after the addition of CCC | Negative screening |
| Negative agglutination after AHG phase and after addition of CCC | The test is invalid and must be repeated |
Use the antigram sheet provided with your specific lot of screening cells to document your test results. These antigram sheets have a reference pattern for each possible antibody based on the cell used. Since screening cells expire within 3-4 weeks, always double-check you’re using the correct panel and sheet.
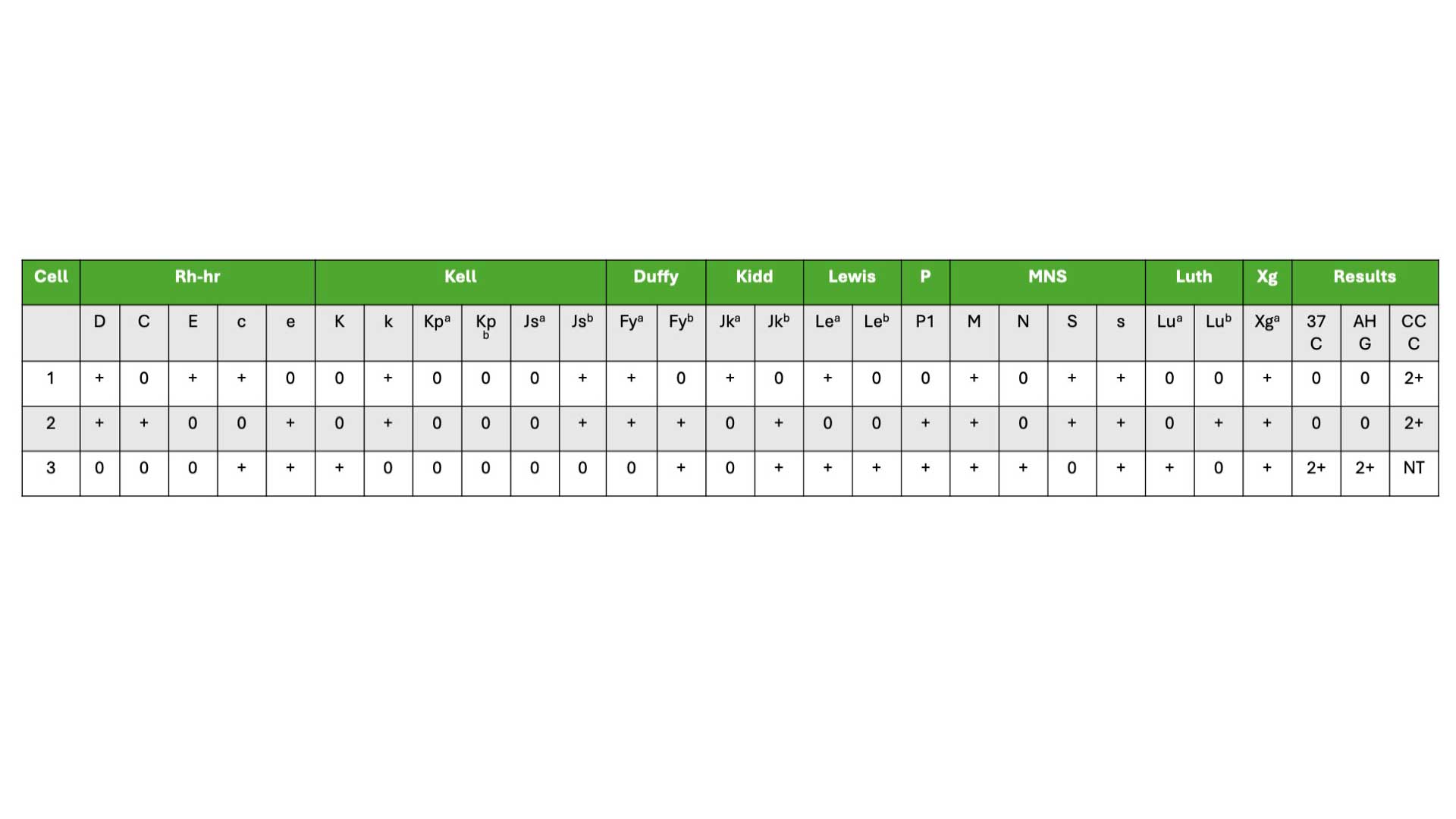
How to read an antigram?
- In the example of the antigram provided above, choose those with negative (0) results in the AHG column. In our example, it will be cell 1 and 2.
- Cross (X) out any positive (+) antigens in cell 1 and 2. For example in cell 1, for Rh antibodies, cross out D, E and c. These means that the patient does not have antibodies against these antigens.
- Circle (0) all remaining antigens which have not been crossed out.
- These findings indicate the possible antibodies present and would require further investigation to identify the specific antibodies present in the patient’s blood.
Additional Considerations
- Quality control procedures are crucial to ensure the accuracy of the test. Positive and negative control red blood cells are typically included to verify the functionality of the reagents and technique.
- If a positive antibody screen is identified, further investigation through antibody identification is necessary to determine the specific antibody and its clinical significance.
Advantages of Antibody Screening
Antibody screening offers a more reliable and sensitive method for detecting red blood cell antibodies compared to serological crossmatching for several reasons:
- Uniform Antigen Expression: Screening cells are typically homozygous for key antigens (e.g., JkaJka genotype for the Jka antigen). This ensures consistent and strong expression of the antigen, allowing even weak antibodies (like anti-Jka) to be readily detected. In contrast, donor red blood cells can be heterozygous (e.g., JkaJkb genotype), potentially leading to weaker antigen expression and potentially missing a weak antibody.
- Preserved Antigen Quality: Screening cells are specifically prepared and stored to optimize antigen preservation. This ensures consistent antigen availability on the cell surface for antibody binding.
- Standardized Technique: Antibody screening is a well-established and standardized process, minimizing the risk of errors compared to manual crossmatching where cell suspensions might be transposed. Additionally, screening offers a more automated and efficient workflow compared to manual crossmatching.
Frequently Asked Questions (FAQs)
What is the rule of 3 in antigram?
The “rule of 3” isn’t specific to certain antigrams, but rather a general observation about how antigrams can be used to interpret antibody screening results. Here’s why:
- Antigens on Screening Cells: Antigrams are reference panels that depict agglutination patterns based on the specific antigens present on the screening cells used in the test.
- Antibody Specificities: Regardless of the antigram design, any antibody can potentially cause agglutination with three different screening cells if the conditions described earlier are met:
- Homozygous antigen expression
- Strong antigen expression on heterozygous cells
- Cross-reactivity with related antigens
Therefore, the “rule of 3” can potentially apply to any antibody specificity as long as the antigram displays agglutination patterns that involve three different screening cells.
Here are some examples:
- Anti-D: If an anti-D antibody is present, it could agglutinate cells with the R1R1 genotype (homozygous D), R1r (heterozygous D with strong expression), and possibly a cell with a related antigen like Du (causing a false positive due to cross-reactivity).
- Anti-Kell (Anti-K): This antibody could agglutinate cells with the KK genotype (homozygous K), Kk (heterozygous K with strong expression), and potentially a cell with a weak K antigen or a related antigen (causing a weaker agglutination due to less efficient binding).
What happens if an antibody screen is positive?
A positive antibody screen result indicates the potential presence of clinically significant unexpected red blood cell (RBC) antibodies in the patient’s plasma or serum. These antibodies can react with antigens on transfused red blood cells, leading to serious complications like hemolytic transfusion reactions.
A positive screen doesn’t tell you the specific antibody. Additional testing, called antibody identification, is crucial. This involves testing the patient’s plasma/serum against a wider panel of red blood cells with defined antigen profiles. By observing the agglutination patterns, the laboratory can identify the specific antibody and its target antigen.
What’s the difference between antibody screening and antibody identification?
Antibody screening acts as a broad net, detecting the potential presence of unexpected red blood cell antibodies in a patient’s blood. It’s a rapid test using a panel of cells with various antigens. A positive screen indicates a potential problem, but doesn’t pinpoint the exact culprit. Antibody identification, on the other hand, is a more detailed investigation. It exposes the patient’s plasma to a wider range of red blood cells with defined antigen profiles. By observing specific agglutination patterns, this test identifies the exact antibody and its target antigen, allowing for selection of truly compatible blood for safe transfusions.
Can your antibody screen change?
Yes, your antibody screen can change over time. Here’s why:
- New Antibody Development: Exposure to foreign red blood cells (through transfusions, pregnancy, or fetomaternal hemorrhage) can trigger the immune system to develop new antibodies.
- Antibody Loss: Some antibodies, particularly IgM type, can weaken or disappear over time.
- Underlying Medical Conditions: Certain diseases can affect antibody production or clearance, potentially leading to changes in your antibody screen results.
Regular antibody screening before transfusions helps ensure safe blood compatibility by detecting any new or existing antibodies.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- American Association of Blood Banks (AABB). Technical Manual, 21st Edition, 2023.
- Dean L. Blood Groups and Red Cell Antigens [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2005.
- Bain BJ, Bates I, Laffan MA. Dacie and Lewis Practical Haematology: Expert Consult: Online and Print 12th Edition (Elsevier). 2016.
- International Society of Blood Transfusion. https://www.isbtweb.org/resources/resources-library.html
- British Committee for Standards in Haematology, C. Milkins, J. Berryman, C. Cantwell, C. Elliott, R. Haggas, J. Jones, M. Rowley, M. Williams, N. Win (2013). Guidelines for pre-transfusion compatibility procedures in blood transfusion laboratories. Transfusion Medicine. 2023(1): 3-35.
- Knowles, S.M., Milkins, C.E., Chapman, J.F. & Scott, M. (2002) The United Kingdom National External Quality Assessment Scheme (blood transfusion laboratory practice): trends in proficiency and practice between 1985 and 2000. Transfusion Medicine, 12, 11–23.
- SHOT (1996–2010) Serious Hazards of Transfusion annual reports. (Accessed 31/05/24).