Procedure-at-a-Glance
This section provides a quick-reference summary to make a peripheral blood smear for a busy laboratory environment.
| Step | Action | Key Tip |
| Preparation | Clean two glass slides with lens paper. | Ensure no fingerprints/dust. |
| Dispensing | Place 1 small drop of EDTA blood 1cm from the end. | Use a consistent drop size (~2-3mm). |
| Positioning | Place spreader slide at a 30° angle in front of the drop. | Angle determines smear thickness. |
| Contact | Pull spreader back until it touches the blood drop. | Let blood spread along the edge. |
| Spreading | Push forward rapidly and smoothly across the slide. | Do not stop mid-stroke. |
| Drying | Air dry or use a hair dryer on “Cool.” | Rapid drying prevents RBC artifacts. |
Quick Checklist for a Perfect Peripheral Blood Smear
- Slide Quality: Are the slides lint-free and grease-free?
- Drop Size: Is the drop roughly 3mm in diameter?
- Timing: Was the smear made within seconds of placing the drop?
- The “Sweep”: Was the motion smooth, fast, and at a consistent 30-45 degree angle?
Introduction to Peripheral Blood Smear
Peripheral blood smears are usually ordered together with a complete blood count. A complete blood count is able to give the quantitative results of the blood cells whereas morphological abnormalities of the blood cells are viewed from the blood smear. Using different stains on the smears allow viewing of the different blood components including inclusion bodies and even parasites.
Principle of Peripheral Blood Smear
The peripheral blood smear, a deceptively simple technique, allows for the visualization of the various blood cells found in whole blood. Its core principle lies in the creation of a thin, uniform film of blood cells spread upon a glass slide. Asmall drop of blood is carefully “smeared” across the surface using another slide held at a specific angle. This spreading action forces the blood cells into a single layer, without overlapping of cells, allowing for individual examination under a microscope. However, simply spreading the blood wouldn’t be enough; uncontrolled spreading would result in distorted and overlapping cells, hindering accurate analysis in the peripheral blood smear.
Materials
- EDTA preserved whole blood
- Glass slide and spreader
- Lens paper
- Hair dryer
Protocol for Peripheral Blood Smear
- Make sure the glass slide and spreader are clean using the lens paper.
- Place 1 drop of well-mixed EDTA blood approximately 1 cm from the end of the glass slide, in the center.
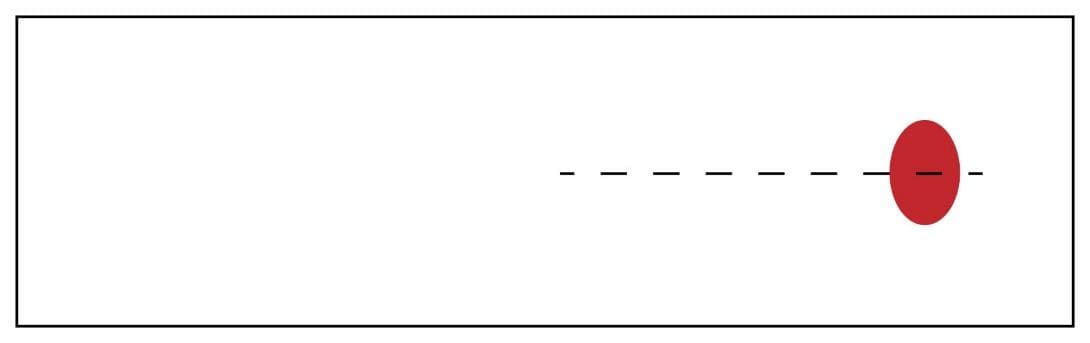
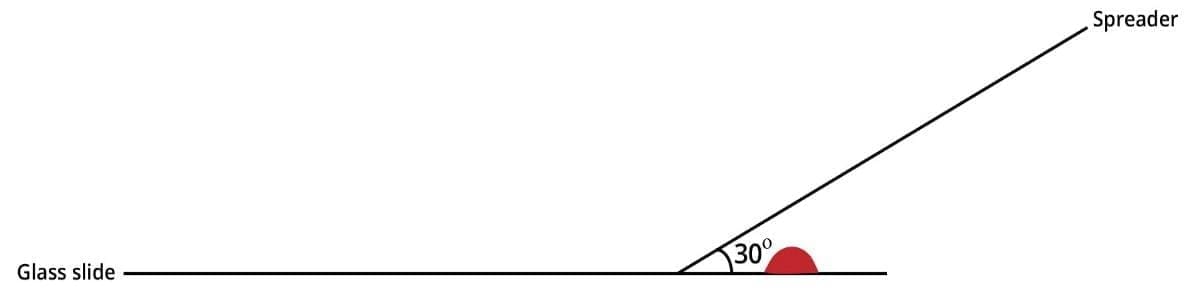
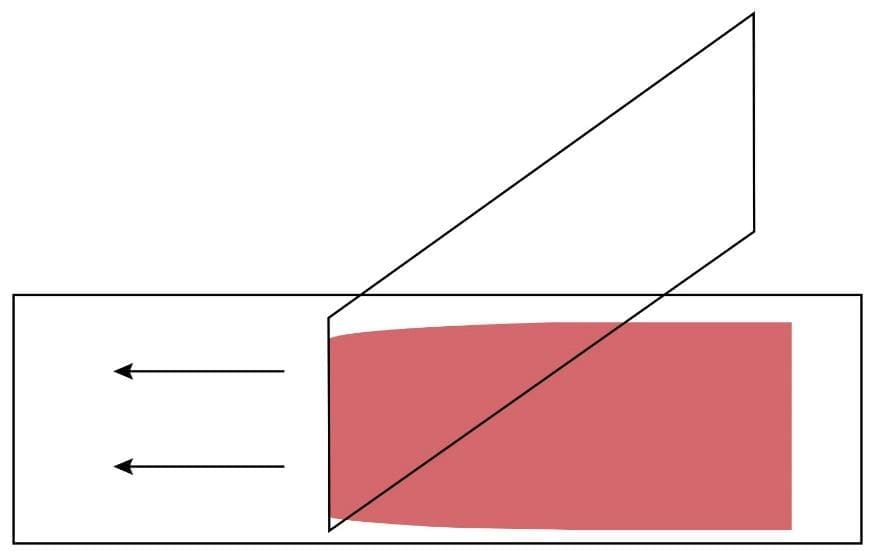
3. Hold the clean spreader at a 30° angle (in relation to the slide) just in front of the blood drop.
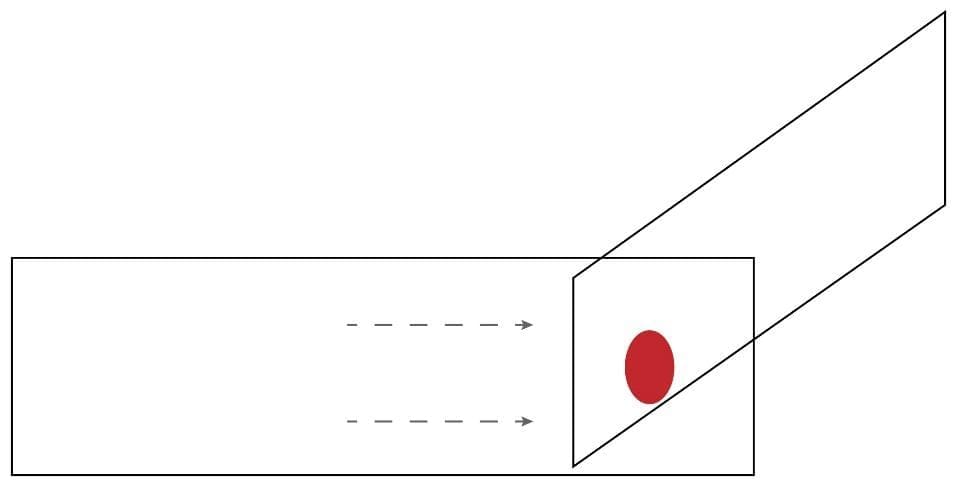
4. Move the spreader backwards to touch the blood drop.
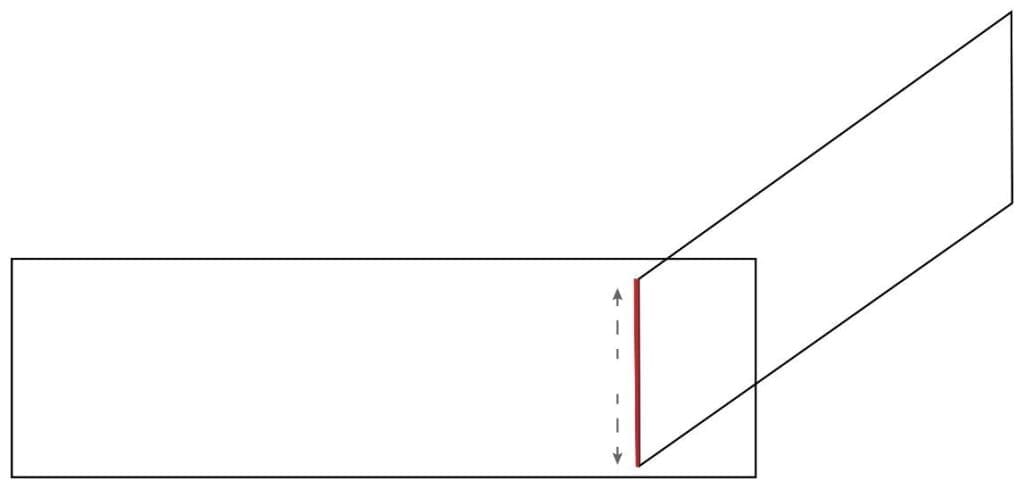
5. Allow the blood to spread the entire width of the spreader.
6. Push the spreader rapidly and smoothly across the glass slide while maintaining the angle of the spreader. The blood should spread across 2/3 of the entire glass slide.
7. Dry the slide using a hair dryer at the lowest speed. The smear can also be air-dried.
8. The blood smear can now be used for staining. Leishman staining protocol can be accessed from our article on Leishman Stain.
Interpretation of Peripheral Blood Smear
- Fast spreading will result in longer and thinner films.
- Angles more than 30° will result in thicker smear.
- Large blood drops will cause the smear to extend beyond the length of the slide.
Troubleshooting Common Peripheral Blood Smear Defects
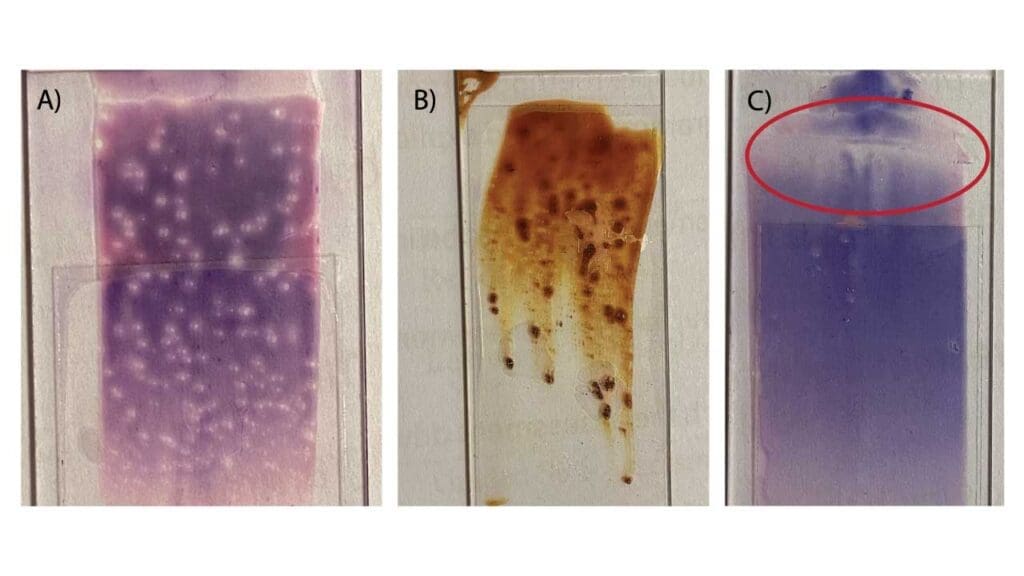
A high-quality blood smear should have a smooth, even appearance with a distinct “feathered edge.” If your smear looks irregular, use the table below to identify the cause and the corrective action.
| Appearance of Smear | Possible Cause | Corrective Action |
| Smear is too short and thick | Spreader slide angle was too steep (high) or the spread was too slow. | Decrease the angle of the spreader slide (~30°) and increase spreading speed. |
| Smear is too long and thin | Spreader slide angle was too shallow (low) or the spread was too fast. | Increase the angle of the spreader slide and decrease spreading speed. |
| Holes or “blank spots” throughout the film | Presence of grease, fingerprints, or dust on the slide. | Use a fresh slide; ensure you only handle slides by the edges. Clean with alcohol if necessary. |
| Long streaks or “tails” at the feathered edge | The blood drop began to dry before spreading, or the spreader slide edge was chipped/dirty. | Spread the drop immediately after dispensing. Use a new, clean spreader slide. |
| Waves or ridges across the smear | Hesitation or “jerky” movement during the spreading stroke. | Maintain a smooth, continuous, and fluid forward motion. |
| Smear is too narrow (does not reach edges) | The blood drop was not allowed to spread across the full width of the spreader slide. | Wait a split second longer for the blood to flow to the edges of the spreader before pushing. |
| “Crenated” Red Blood Cells (Artifacts) | The smear dried too slowly (often due to high humidity). | Use a fan or hair dryer on a “cool” setting to speed up the drying process. |
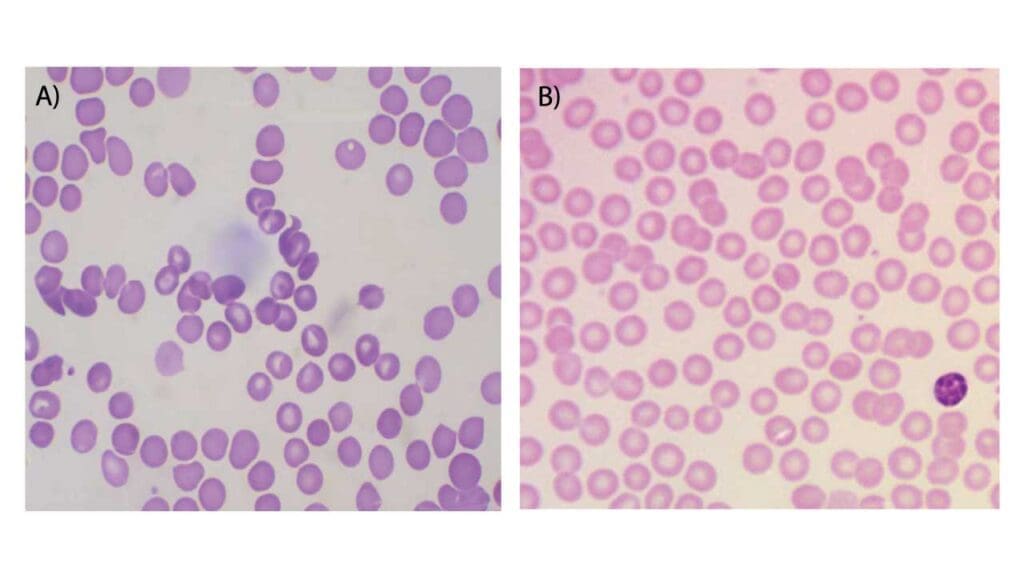
| Smudged White Blood Cells | Excessive pressure on the spreader slide or the blood is “old” (EDTA > 24 hours). | Use a lighter touch; always use fresh blood (less than 2–4 hours old). |
Frequently Asked Questions (FAQs)
How soon should a smear be made after blood collection?
Ideally within 2 hours. If blood sits in EDTA for too long (over 4-5 hours), white blood cells may develop vacuoles and red cells may undergo “crenation” (shrinking and becoming spiky).
Why is my peripheral blood smear too short and thick?
This is usually caused by using a spreader angle that is too steep (greater than 45°) or moving the spreader slide too slowly. Increasing the speed and lowering the angle will result in a longer, thinner smear.
Can I use finger-prick (capillary) blood for a peripheral blood smear?
Yes, but you must work quickly to avoid clotting. The first drop of blood should be wiped away, and the second drop used for the peripheral blood smear.
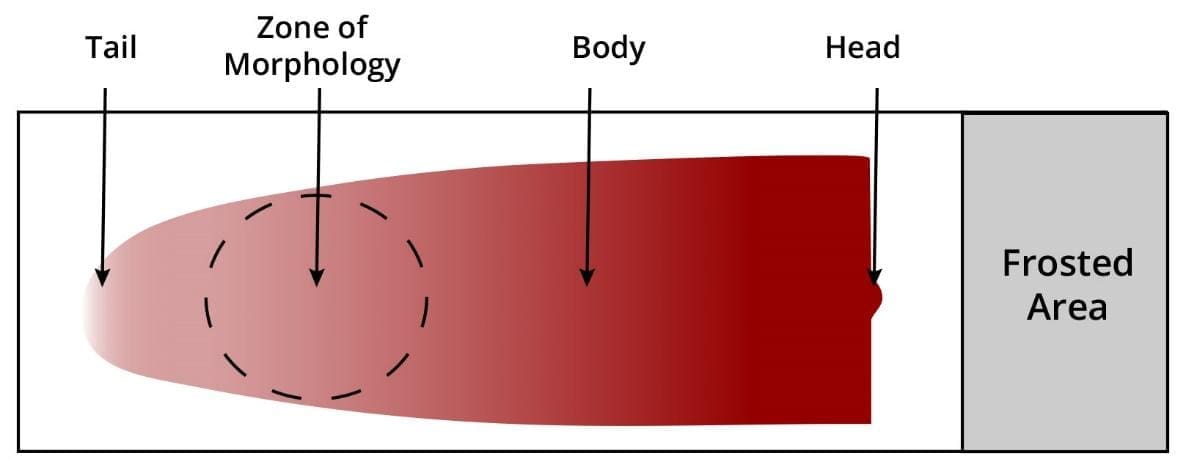
What is the “Zone of Morphology”?
This is the area just behind the feathered edge on the peripheral blood smear. In this region, approximately 50% of the RBCs should be slightly overlapping, and the other 50% should be separate, allowing for accurate identification of cell shapes and inclusions.
Glossary of Related Medical Terms
- EDTA (Ethylenediaminetetraacetic acid): The standard anticoagulant for hematology that preserves cell morphology by chelating calcium.
- Feathered Edge: The thin end of the blood smear where cells are spread out enough to be viewed individually.
- Monolayer: The specific “Zone of Morphology” where red blood cells are side-by-side without overlapping or touching.
- Wedge Technique: The manual method of preparing a smear using a spreader slide at an angle.
Disclaimer: This protocol is intended for informational purposes only and may need to be modified depending on the specific laboratory procedures and patient circumstances. Always consult with a qualified healthcare professional for guidance. See additional information.
References
- Bain BJ. A Practical Guide. 6th Edition (Wiley). 2022.
- Bain BJ, Bates I, Laffan MA. Dacie and Lewis Practical Haematology: Expert Consult: Online and Print 12th Edition (Elsevier). 2016.
- Lynch EC. Peripheral Blood Smear. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 155. Available from: https://www.ncbi.nlm.nih.gov/books/NBK263/
- Adewoyin, A. S., & Nwogoh, B. (2014). Peripheral blood film – a review. Annals of Ibadan postgraduate medicine, 12(2), 71–79.



